- Record: found
- Abstract: found
- Article: found
New therapies for the treatment of heart failure: a summary of recent accomplishments
Abstract
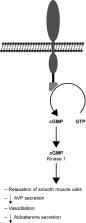
Despite continuous efforts to prevent cardiovascular diseases (CVDs), heart failure prevails as the number one cause of death in developed countries. To properly treat CVDs, scientists had to take a closer look at the factors that contribute to their pathogenesis and either modernize current pharmaceuticals or develop brand new treatments. Enhancement of current drugs, such as tolvaptan and omecamtiv mecarbil, sheds new light on already-known therapies. Tolvaptan, a vasopressin antagonist, could be adopted in heart failure therapy as it reduces pre- and afterload by decreasing systolic blood pressure and blood volume. Omecamtiv mecarbil, which is a myosin binding peptide, could aid cardiac contractility. The next generation vasodilators, serelaxin and ularitide, are based on naturally occurring peptides and they reduce peripheral vascular resistance and increase the cardiac index. In combination with their anti-inflammatory properties, they could turn out to be extremely potent drugs for heart failure treatment. Cardiotrophin has exceeded many researchers’ expectations, as evidence suggests that it could cause sarcomere hypertrophy without excessive proliferation of connective tissue. Rapid progress in gene therapy has caused it to finally be considered as one of the viable options for the treatment of CVDs. This novel therapeutic approach could restore stable heart function either by restoring depleted membrane proteins or by balancing the intracellular calcium concentration. Although it has been set back by problems concerning its long-term effects, it is still highly likely to succeed.
Most cited references49
- Record: found
- Abstract: not found
- Article: not found
2016 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure: The Task Force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC). Developed with the special contribution of the Heart Failure Association (HFA) of the ESC.
- Record: found
- Abstract: found
- Article: not found
Serelaxin, recombinant human relaxin-2, for treatment of acute heart failure (RELAX-AHF): a randomised, placebo-controlled trial.
- Record: found
- Abstract: found
- Article: not found