- Record: found
- Abstract: found
- Article: found
Cerebral Organoids Repair Ischemic Stroke Brain Injury
Read this article at
Abstract
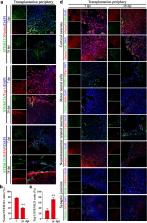
Stroke is the second leading cause of death and main cause of disability worldwide, but with few effective therapies. Although stem cell-based therapy has been proposed as an exciting regenerative medicine strategy for brain injury, there are limitations. The developed cerebral organoids (COs) represent a promising transplantation source for stroke that remains to be answered. Here, we transplanted COs at 55 days and explored the feasibility in the rat middle cerebral artery occlusion (MCAO) model of stroke. COs transplantation at 6 h or even 24 h after MCAO significantly reduces brain infarct volume and improves neurological motor function. Transplanted COs show the potential of multilineage differentiation to mimic in vivo cortical development, support motor cortex region-specific reconstruction, form neurotransmitter-related neurons, and achieve synaptic connection with host brain via in situ differentiation and cell replacement in stroke. Cells from transplanted COs show extensive migration into different brain regions along corpus callosum. The mechanisms underlying COs transplantation therapy are also associated with enhanced neurogenesis, synaptic reconstruction, axonal regeneration and angiogenesis, and decreased neural apoptosis with more survival neurons after stroke. Moreover, COs transplantation promotes predominantly exogenous neurogenesis in the transplantation periphery of ipsilateral cortex and predominantly endogenous neurogenesis in the hippocampus and subventricular zone. Together, we demonstrate the efficacy and underlying mechanisms of COs transplantation in stroke. This preliminary but promising study provides first-hand preclinical evidence for COs transplantation as a potential and effective intervention for stroke treatment.
Related collections
Most cited references24
- Record: found
- Abstract: found
- Article: not found
Young dentate granule cells mediate pattern separation, whereas old granule cells facilitate pattern completion.

- Record: found
- Abstract: found
- Article: not found