- Record: found
- Abstract: found
- Article: found
A Cell-Permeant Mimetic of NMN Activates SARM1 to Produce Cyclic ADP-Ribose and Induce Non-apoptotic Cell Death

Read this article at
Summary
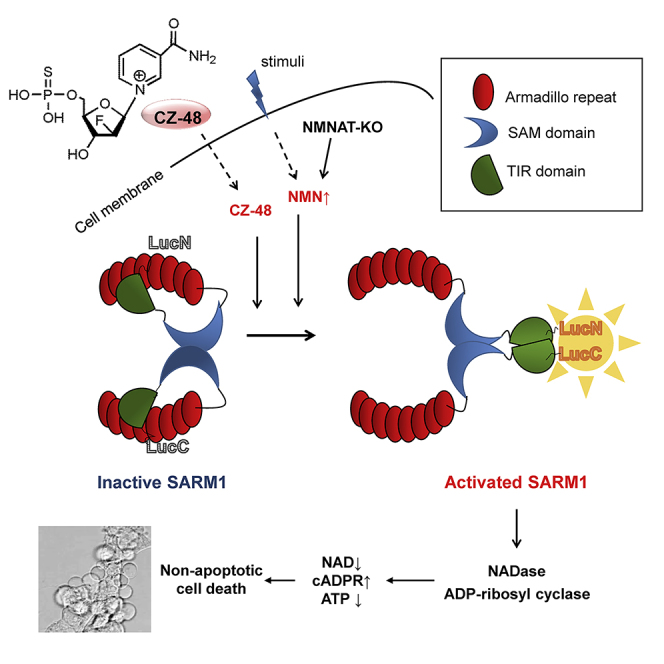
SARM1, an NAD-utilizing enzyme, regulates axonal degeneration. We show that CZ-48, a cell-permeant mimetic of NMN, activated SARM1 in vitro and in cellulo to cyclize NAD and produce a Ca 2+ messenger, cADPR, with similar efficiency as NMN. Knockout of NMN-adenylyltransferase elevated cellular NMN and activated SARM1 to produce cADPR, confirming NMN was its endogenous activator. Determinants for the activating effects and cell permeability of CZ-48 were identified. CZ-48 activated SARM1 via a conformational change of the auto-inhibitory domain and dimerization of its catalytic domain. SARM1 catalysis was similar to CD38, despite having no sequence similarity. Both catalyzed similar set of reactions, but SARM1 had much higher NAD-cyclizing activity, making it more efficient in elevating cADPR. CZ-48 acted selectively, activating SARM1 but inhibiting CD38. In SARM1-overexpressing cells, CZ-48 elevated cADPR, depleted NAD and ATP, and induced non-apoptotic death. CZ-48 is a specific modulator of SARM1 functions in cells.
Graphical Abstract
Highlights
-
•
CZ-48, a cell-permeant mimetic of NMN, activates SARM1 but inhibits CD38 enzymatically
-
•
SARM1 catalysis is similar to CD38, but with higher cyclase activity
-
•
Activation by CZ-48 or NMN elicits conformational changes in SARM1
-
•
Activation of SARM1 causes cADPR production, NAD depletion, and non-apoptotic cell death
Abstract
Biochemistry; Enzymology; Biochemical Mechanism
Related collections
Most cited references53
- Record: found
- Abstract: found
- Article: not found
TAL effectors: customizable proteins for DNA targeting.
- Record: found
- Abstract: found
- Article: not found
Evolution and function of the ADP ribosyl cyclase/CD38 gene family in physiology and pathology.
- Record: found
- Abstract: found
- Article: not found