- Record: found
- Abstract: found
- Article: found
The Actin Cytoskeleton Is Involved in Glial Cell Line-Derived Neurotrophic Factor (GDNF)-Induced Ret Translocation into Lipid Rafts in Dopaminergic Neuronal Cells
Read this article at
Abstract
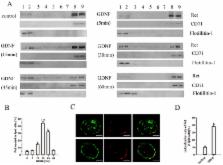
Glial cell line-derived neurotrophic factor (GDNF), a potential therapeutic factor for Parkinson’s disease (PD), exerts its biological effects through the Ret receptor tyrosine kinase. The redistribution of Ret into lipid rafts substantially influences Ret signaling, but the mechanisms underlying Ret translocation remain unclear. The purpose of our study was to further explore the signaling mechanisms of GDNF and to determine whether the actin cytoskeleton is involved in the GDNF-induced Ret translocation into lipid rafts. In MN9D dopaminergic neuronal cells, we used density gradient centrifugation and immunofluorescence confocal microscopy to separate and visualize lipid rafts, co-immunoprecipitation to analyze protein-protein interactions, and latrunculin B (Lat B) and jasplakinolide (Jas) to disrupt and enhance the polymerization of the actin cytoskeleton, respectively. The results showed that Ret translocated into lipid rafts and coimmunoprecipitated with actin in response to GDNF treatment. After Lat B or Jas treatment, the Ret–F-actin association induced by GDNF was impaired or enhanced respectively and then the levels of Ret translocated into lipid rafts were correspondingly inhibited or promoted. These data indicate that actin polymerization and cytoskeletal remodeling are integral to GDNF-induced cell signaling in dopaminergic cells and define a new role of the actin cytoskeleton in promoting Ret redistribution into lipid rafts.
Related collections
Most cited references18
- Record: found
- Abstract: found
- Article: not found
Active remodeling of cortical actin regulates spatiotemporal organization of cell surface molecules.
- Record: found
- Abstract: found
- Article: not found
Aggregation of Lipid Rafts Accompanies Signaling via the T Cell Antigen Receptor
- Record: found
- Abstract: found
- Article: not found