- Record: found
- Abstract: found
- Article: found
Effects of carrier solutions on the viability and efficacy of canine adipose-derived mesenchymal stem cells
Read this article at
Abstract
Background
Mesenchymal stem cells (MSCs) have favorable characteristics that render them a potent therapeutic tool. We tested the characteristics of MSCs after temporal storage in various carrier solutions, such as 0.9% saline (saline), 5% dextrose solution (DS), heparin in saline, and Hartmann’s solution, all of which are approved by the U.S. Food and Drug Administration (FDA). Phosphate-buffered saline, which does not have FDA approval, was also used as a carrier solution. We aimed to examine the effects of these solutions on the viability and characteristics of MSCs to evaluate their suitability and efficacy for the storage of canine adipose-derived MSCs (cADMSCs).
Results
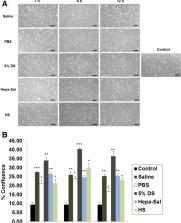
We stored the cADMSCs in the test carrier solutions in a time-dependent manner (1, 6, and 12 h) at 4 °C, and analyzed cell confluency, viability, proliferation, self-renewability, and chondrogenic differentiation. Cell confluency was significantly higher in 5% DS and lower in phosphate-buffered saline at 12 h compared to other solutions. cADMSCs stored in saline for 12 h showed the highest viability rate. However, at 12 h, the proliferation rate of cADMSCs was significantly higher after storage in 5% DS and significantly lower after storage in saline, compared to the other solutions. cADMSCs stored in heparin in saline showed superior chondrogenic capacities at 12 h compared to other carrier solutions. The expression levels of the stemness markers, Nanog and Sox2, as well as those of the MSC surface markers, CD90 and CD105, were also affected over time.
Conclusion
Our results suggest that MSCs should be stored in saline, 5% DS, heparin in saline, or Hartmann’s solution at 4 °C, all of which have FDA approval (preferable storage conditions: less than 6 h and no longer than 12 h), rather than storing them in phosphate-buffered saline to ensure high viability and efficacy.
Related collections
Most cited references75
- Record: found
- Abstract: found
- Article: not found
Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method.
- Record: found
- Abstract: found
- Article: not found
Adult mesenchymal stem cells for tissue engineering versus regenerative medicine.
- Record: found
- Abstract: found
- Article: not found