- Record: found
- Abstract: found
- Article: not found
Ertugliflozin in Patients with Stage 3 Chronic Kidney Disease and Type 2 Diabetes Mellitus: The VERTIS RENAL Randomized Study
Read this article at
Abstract
Introduction
Ertugliflozin is a sodium-glucose cotransporter 2 inhibitor in development for type 2 diabetes mellitus (T2DM). The safety and efficacy of ertugliflozin were evaluated over 52 weeks in patients with chronic kidney disease (CKD).
Methods
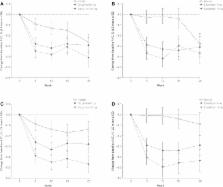
In this double-blind randomized study (NCT01986855), patients with glycated hemoglobin (A1C) 7.0–10.5% and stage 3 CKD [estimated glomerular filtration rate (eGFR) ≥ 30 to < 60 mL/min/1.73 m 2] who were undergoing treatment with standard diabetes therapy (or therapies) including insulin and/or sulfonylureas were randomized to once-daily ertugliflozin 5 mg, 15 mg, or placebo. Patients on metformin underwent a pre-randomization ≥ 10-week wash-off period. The primary endpoint was change from baseline in A1C at week 26 in the overall cohort. Secondary efficacy endpoints were assessed in the stage 3A CKD cohort (eGFR ≥ 45 to < 60 mL/min/1.73 m 2) at weeks 26 and 52. Safety was assessed in the overall cohort.
Results
468 patients were randomized (baseline mean A1C 8.2%). At week 26, reductions from baseline in A1C were observed across groups in the overall cohort [least squares mean changes (95% confidence interval) – 0.3% (– 0.4, – 0.1), – 0.3% (– 0.4, – 0.1), and – 0.4% (– 0.6, – 0.3) for placebo and for ertugliflozin 5 mg and 15 mg, respectively]. Prohibited use of metformin was identified in ~ 17% of patients and impacted evaluation of the primary endpoint. Greater reductions from baseline in body weight, fasting plasma glucose, and systolic blood pressure were observed with ertugliflozin versus placebo at week 26 (stage 3A CKD cohort). The incidences of urinary tract infections, genital mycotic infections, and hypoglycemia adverse events were not meaningfully different between groups. The incidence of hypovolemia-related adverse events was higher with ertugliflozin relative to placebo.
Conclusion
Although surreptitious metformin use impacted the primary analysis, reductions in blood glucose and body weight were observed with ertugliflozin in patients with T2DM and stage 3 CKD; ertugliflozin had an acceptable safety profile.
Related collections
Most cited references10

- Record: found
- Abstract: found
- Article: found
Sodium-Glucose Cotransport Inhibition With Dapagliflozin in Type 2 Diabetes

- Record: found
- Abstract: found
- Article: found
Efficacy and safety of canagliflozin in subjects with type 2 diabetes and chronic kidney disease
- Record: found
- Abstract: not found
- Article: not found
