- Record: found
- Abstract: found
- Article: found
Skeletal muscle contraction. The thorough definition of the contractile event requires both load acceleration and load mass to be known
Read this article at
Abstract
Background
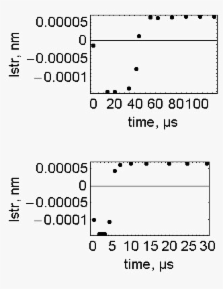
The scope of this work is to show that the correct and complete definition of the system of muscle contraction requires the knowledge of both the mass and the acceleration of the load.
Results
The aim is achieved by making use of a model of muscle contraction that operates into two phases. The first phase considers the effects of the power stroke in the absence of any hindrance. In the second phase viscous hindrance is introduced to match the experimental speed and yield of the contraction. It is shown that, at constant force of the load, changing load acceleration changes the time course of the pre-steady state of myofibril contraction. The decrease of the acceleration of the load from 9.8 m.s -2 to 1 m.s -2 increases the time length of the pre-steady state of the contraction from a few microseconds to many hundreds of microseconds and decreases the stiffness of the active fibre.
Related collections
Most cited references19
- Record: found
- Abstract: not found
- Article: not found
Preparation of myosin and its subfragments from rabbit skeletal muscle.
- Record: found
- Abstract: found
- Article: not found