- Record: found
- Abstract: found
- Article: not found
The eye as a complement dysregulation hotspot
Read this article at
Abstract
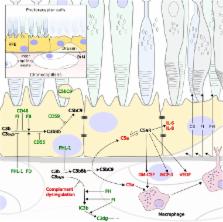
Complement turnover is tightly regulated throughout the human body in order to prevent over-activation and subsequent damage from inflammation. In the eye, low-level complement activation is maintained to provide immune tolerance in this immune privileged organ. Conversely, the complement system is suppressed in the cornea to protect it from continuous immunological insult. Over-activation of the complement cascade has been implicated in the disease progression of glaucoma and diabetic retinopathy and is now known to be a central driver in the pathogenesis of age-related macular degeneration (AMD). Indeed, it is with AMD where the most recent and exciting work has been carried out with complement-based therapies entering into clinical trials. However, the success of these trials will depend upon delivering the therapeutics to the correct anatomical sites within the eye, so a full understanding of how complement regulation is compartmentalized in the eye is required, a topic that will be highlighted in this review.
Related collections
Most cited references59
- Record: found
- Abstract: found
- Article: not found
The pivotal role of the complement system in aging and age-related macular degeneration: hypothesis re-visited.
- Record: found
- Abstract: found
- Article: not found
The dynamic nature of Bruch's membrane.
- Record: found
- Abstract: found
- Article: not found
