- Record: found
- Abstract: found
- Article: found
Non-Coding RNAs and Their Role in Respiratory Syncytial Virus (RSV) and Human Metapneumovirus (hMPV) Infections
Read this article at
Abstract
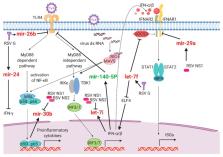
Recent high-throughput sequencing revealed that only 2% of the transcribed human genome codes for proteins, while the majority of transcriptional products are non-coding RNAs (ncRNAs). Herein, we review the current knowledge regarding ncRNAs, both host- and virus-derived, and their role in respiratory syncytial virus (RSV) and human metapneumovirus (hMPV) infections. RSV is known as the most common cause of lower respiratory tract infection (LRTI) in children, while hMPV is also a significant contributor to LRTI in the pediatrics population. Although RSV and hMPV are close members, belonging to the Pneumoviridae family, they induce distinct changes in the ncRNA profile. Several types of host ncRNAs, including long ncRNA (lncRNA), microRNAs (miRNAs), and transfer RNA (tRNA)-derived RNA fragments (tRFs), are involved as playing roles in RSV and/or hMPV infection. Given the importance of ncRNAs in regulating the expression and functions of genes and proteins, comprehensively understanding the roles of ncRNAs in RSV/hMPV infection could shed light upon the disease mechanisms of RSV and hMPV, potentially providing insights into the development of prevention strategies and antiviral therapy. The presence of viral-derived RNAs and the potential of using ncRNAs as diagnostic biomarkers are also discussed in this review.
Related collections
Most cited references77
- Record: found
- Abstract: found
- Article: not found
Asthma and allergy patterns over 18 years after severe RSV bronchiolitis in the first year of life.
- Record: found
- Abstract: found
- Article: not found
Severe respiratory syncytial virus bronchiolitis in infancy and asthma and allergy at age 13.
- Record: found
- Abstract: found
- Article: not found