- Record: found
- Abstract: found
- Article: found
Rutin and Its Combination With Inulin Attenuate Gut Dysbiosis, the Inflammatory Status and Endoplasmic Reticulum Stress in Paneth Cells of Obese Mice Induced by High-Fat Diet
Read this article at
Abstract
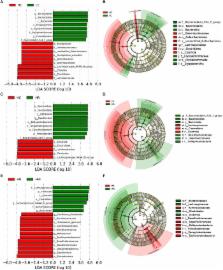
Gut dysbiosis induced by high fat diet (HF) or obesity is a predisposing factor to develop diverse inflammatory diseases. Polyphenols and fibers, often eaten together, have been reported to have prebiotic actions, but their health promoting benefits still need to be further characterized and defined. This study attempted to understand how polyphenol rutin and polysaccharide inulin influence intestinal health in mouse model fed a HF (60 kcal%) diet. A total of 48 C57BL/6J mice were divided into four groups fed with a low fat (10% kcal%) control diet (LC), a high fat control diet (HC), a high-fat diet supplemented with rutin (HR), or a high-fat diet supplemented rutin and inulin (HRI) for 20 weeks. Rutin supplementation reduced the HF diet-induced increase of Firmicutes/ Bacteroidetes (F/B) ratio, Deferribacteraceae population and plasma lipopolysaccharide (LPS) ( p < 0.05); ameliorated inflammation as indicated by the decreased circulating inflammatory cytokines ( p < 0.05) and the reduced expressions of intestinal inflammatory mediators ( p < 0.05); and attenuated the endoplasmic reticulum (ER) stress in Paneth cells as indicated by the decreased expressions of the ER markers ( p < 0.05). Compared to the rutin supplementation alone, the co-administration of rutin with inulin improved the utilization of rutin as indicated by its decreased excretion, suppressed a number of harmful bacteria including Deferribacteraceae and Desulfovibrionaceae ( p < 0.05), and further reduced the expression of the key inflammatory cytokine TNF-α and increased the production of butyrate, despite the supplementation of inulin reversed the decrease of body weight induced by rutin supplementation due to an increased food intake. Taken together, our data demonstrated that rutin supplementation ameliorated the inflammatory status and ER stress in Paneth cells under a HF-induced obese state, and its co-administration with inulin further mitigated the inflammatory status, indicating the potential to combine polyphenol rutin and the polysaccharide inulin as a dietary strategy to ameliorate gut dysbiosis, to improve inflammatory status and thereby to reduce medical disorders associated with HF-induced obesity.
Related collections
Most cited references32
- Record: found
- Abstract: found
- Article: not found
Interaction between phenolics and gut microbiota: role in human health.
- Record: found
- Abstract: found
- Article: not found
Dietary Polyphenols Promote Growth of the Gut Bacterium Akkermansia muciniphila and Attenuate High-Fat Diet–Induced Metabolic Syndrome
- Record: found
- Abstract: found
- Article: not found