- Record: found
- Abstract: found
- Article: found
Evaluation of the Effects of Pasireotide LAR Administration on Lymphocele Prevention after Axillary Node Dissection for Breast Cancer: Results of a Randomized Non-Comparative Phase 2 Study
Read this article at
Abstract
Objective
The aim of this study was to assess the efficacy (response rate centered on 80%) of a somatostatin analog with high affinity for 4 somatostatin receptors in reducing the postoperative incidence of symptomatic lymphocele formation following total mastectomy with axillary lymph node dissection.
Setting
This prospective, double-blind, randomised, placebo-controlled, phase 2 trial was conducted in two secondary care centres.
Participants
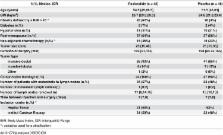
All female patients for whom mastectomy and axillary lymph node dissection were indicated were eligible for the study, including patients who had received neo-adjuvant chemotherapy. Main exclusion criteria were related to diabetes, cardiac insufficiency, disorder of cardiac conduction or hepatic failure.
Interventions
Patients were randomised to receive one injection of either prolonged-release pasireotide 60 mg or placebo (physiological serum), which were administered intramuscularly 7 to 10 days before the scheduled surgery. The study was conducted in a double-blind manner.
Primary and Secondary Outcome Measures
The primary outcome measure was the percentage of patients who did not develop post-operative axillary symptomatic lymphoceles during the 2 postoperative months. Secondary endpoints were the total quantity of lymph drained, duration and daily volume of drainage and aspirated volumes of lymph.
Results
Ninety-one patients were randomised. Ninety patients were evaluable: 42 patients received pasireotide, and 48 patients received placebo. The mean estimated response rate were 62.4% (95% Credibility Interval [CrI]: 48.6%-75.3%) in the treatment group and 50.2% (95% CrI: 37.6%-62.8%) in the placebo group. Overall safety was comparable across groups, and one serious adverse event occurred. In the treatment group, one patient with known insulin-depe*ndent diabetes required hospitalization for hyperglycaemia.
Conclusions
With this phase 2 preliminary study, even if our results indicate a trend towards a reduction in symptomatic lymphocele, pre-operative injection of pasireotide failed to achieve a response rate centered on 80%. Pharmacokinetics analysis suggests that effect of pasireotide could be optimised.
Related collections
Most cited references23
- Record: found
- Abstract: found
- Article: not found
A 12-month phase 3 study of pasireotide in Cushing's disease.
- Record: found
- Abstract: found
- Article: not found
SOM230: a novel somatostatin peptidomimetic with broad somatotropin release inhibiting factor (SRIF) receptor binding and a unique antisecretory profile.
- Record: found
- Abstract: found
- Article: not found