- Record: found
- Abstract: found
- Article: found
Protocol for the rapid intravenous in ovo injection of developing amniote embryos

Read this article at
Summary
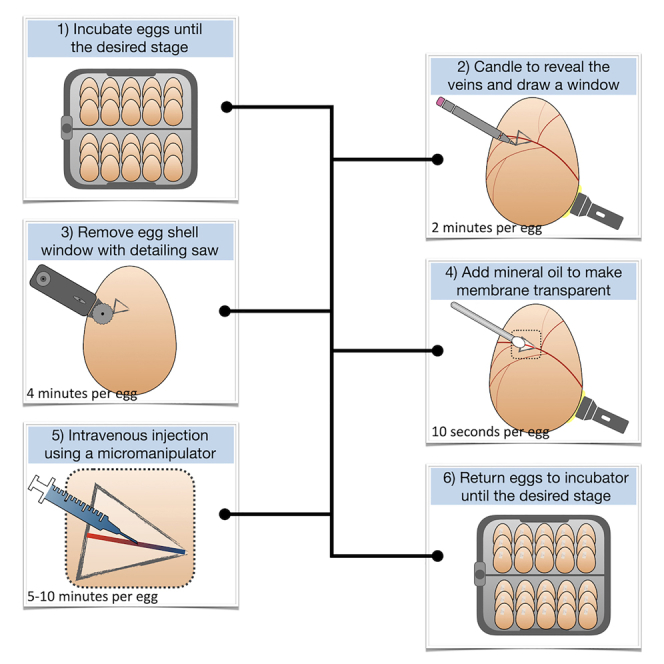
We present a technique for precise drug delivery into the vascular system of developing amniote embryos via injection into chorioallantoic veins underlying the eggshell membrane. We describe steps for incubating and candling eggs, removing the shell to expose underlying veins, and precise intravenous injection. In addition to chicken embryos, this protocol is applicable to other amniote species that lay hard-shell eggs, including crocodiles and tortoises. This technique is rapid, is reproducible, is of low cost, and will provide an important resource for developmental biologists.
For complete details on the use and execution of this protocol, please refer to Cooper & Milinkovitch. 1
Graphical abstract
Highlights
-
•
Technique for the intravenous injection of amniote embryos within hard-shelled eggs
-
•
Description of experimental manipulation of chicken, crocodile, and tortoise eggs
-
•
Protocol is precise, fast, and of low cost, making it widely accessible to researchers
-
•
Broadly applicable to studies in developmental biology and beyond
Abstract
Publisher’s note: Undertaking any experimental protocol requires adherence to local institutional guidelines for laboratory safety and ethics.
Abstract
We present a technique for precise drug delivery into the vascular system of developing amniote embryos via injection into chorioallantoic veins underlying the eggshell membrane. We describe steps for incubating and candling eggs, removing the shell to expose underlying veins, and precise intravenous injection. In addition to chicken embryos, this protocol is applicable to other amniote species that lay hard-shell eggs, including crocodiles and tortoises. This technique is rapid, is reproducible, is of low-cost, and will provide an important resource for developmental biologists.
Related collections
Most cited references13
- Record: found
- Abstract: found
- Article: not found
Distinct Wnt members regulate the hierarchical morphogenesis of skin regions (spinal tract) and individual feathers.
- Record: found
- Abstract: found
- Article: not found
Multiple Regulatory Modules Are Required for Scale-to-Feather Conversion
- Record: found
- Abstract: found
- Article: not found