- Record: found
- Abstract: found
- Article: found
The antipyretic efficacy and safety of propacetamol compared with dexibuprofen in febrile children: a multicenter, randomized, double-blind, comparative, phase 3 clinical trial
Read this article at
Abstract
Background
We aimed to compare the antipyretic efficacy, safety, and tolerability between oral dexibuprofen and intravenous propacetamol in children with upper respiratory tract infection (URTI) presenting with fever.
Methods
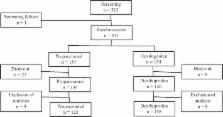
Patients aging from 6 months to 14 years admitted for URTI with axillary body temperature ≥ 38.0 °C were enrolled and randomized into the study or control group. Patients in the study group were intravenously infused with propacetamol and subsequently oral placebo medication was administered. Patients in the control group were intravenously infused with 100 mL of 0.9% sodium chloride solution without propacetamol and then oral dexibuprofen was administered. We checked the body temperature of all patients at 0.5 h (hr), 1 h, 1.5 h, 2 h, 3 h, 4 h, and 6 h after oral placebo or dexibuprofen had been applied.
Results
A total of 263 patients (125 in the study group) were finally enrolled. The body temperatures of patients in the study group were significantly lower until 2 h after administration (37.73 ± 0.58 vs 38.36 ± 0.69 °C ( p < 0.001), 37.37 ± 0.53 vs 37.88 ± 0.69 °C ( p < 0.001), 37.27 ± 0.60 vs 37.62 ± 0.66 °C ( p < 0.001), 37.25 ± 0.62 vs 37.40 ± 0.60 °C ( p = 0.0452), at 0.5 h, 1 h, 1.5 h, and 2 h, respectively). The two groups showed no significant differences in terms of the range of body temperature decrease, the Area Under the Curve of body temperature change for antipyretic administration-and-time relationship, the maximum value of body temperature decrease during the 6 h test period, the number of patients whose body temperature normalized (< 37.0 °C), the mean time when first normalization of body temperature, and the development of adverse events including gastrointestinal problem, elevated liver enzyme, and thrombocytopenia.
Related collections
Most cited references27
- Record: found
- Abstract: found
- Article: not found
Fever phobia revisited: have parental misconceptions about fever changed in 20 years?
- Record: found
- Abstract: found
- Article: not found
Comparative study of the efficacy and safety of paracetamol, ibuprofen, and indomethacin in closure of patent ductus arteriosus in preterm neonates.
- Record: found
- Abstract: found
- Article: not found