- Record: found
- Abstract: found
- Article: found
LMOf2365_0442 Encoding for a Fructose Specific PTS Permease IIA May Be Required for Virulence in L. monocytogenes Strain F2365
Read this article at
Abstract
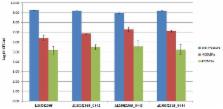
Listeria monocytogenes is a foodborne pathogen that causes listeriosis, which is a major public health concern due to the high fatality rate. LMOf2365_0442, 0443, and 0444 encode for fructose-specific EIIABC components of phosphotransferase transport system (PTS) permease that is responsible for sugar transport. In previous studies, in-frame deletion mutants of a putative fructose-specific PTS permease ( LMOf2365_0442, 0443, and 0444) were constructed and analyzed. However, the virulence potential of these deletion mutants has not been studied. In this study, two in vitro methods were used to analyze the virulence potential of these L. monocytogenes deletion mutants. First, invasion assays were used to measure the invasion efficiencies to host cells using the human HT-29 cell line. Second, plaque forming assays were used to measure cell-to-cell spread in host cells. Our results showed that the deletion mutant Δ LMOf2365_0442 had reduced invasion and cell-to-cell spread efficiencies in human cell line compared to the parental strain LMOf2365, indicating that LMOf2365_0442 encoding for a fructose specific PTS permease IIA may be required for virulence in L. monocytogenes strain F2365. In addition, the gene expression levels of 15 virulence and stress-related genes were analyzed in the stationary phase cells of the deletion mutants using RT-PCR assays. Virulence-related gene expression levels were elevated in the deletion mutants Δ LMOf2365_0442-0444 compared to the wild type parental strain LMOf2365, indicating the down-regulation of virulence genes by this PTS permease in L. monocytogenes. Finally, stress-related gene clpC expression levels were also increased in all of the deletion mutants, suggesting the involvement of this PTS permease in stress response. Furthermore, these deletion mutants displayed the same pressure tolerance and the same capacity for biofilm formation compared to the wild-type parental strain LMOf2365. In summary, our findings suggest that the LMOf2365_0442 gene can be used as a potential target to develop inhibitors for new therapeutic and pathogen control strategies for public health.
Related collections
Most cited references30
- Record: found
- Abstract: found
- Article: not found
Bacteriocin-based strategies for food biopreservation.
- Record: found
- Abstract: found
- Article: not found
The mechanisms of carbon catabolite repression in bacteria.
- Record: found
- Abstract: found
- Article: not found