- Record: found
- Abstract: found
- Article: found
Early versus late initiation of renal replacement therapy in critically ill patients with acute kidney injury (The ELAIN-Trial): study protocol for a randomized controlled trial
Read this article at
Abstract
Background
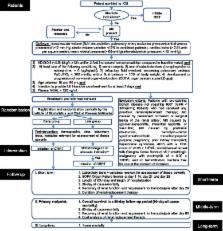
Acute kidney injury remains a common complication in critically ill patients and despite multiple trials and observational studies, the optimal timing for initiation of renal replacement therapy is still unclear. The early versus late initiation of renal replacement therapy in critically ill patients with acute kidney injury (ELAIN) study is a randomized, single-center, prospective, two-arm, parallel group trial to reduce mortality in patients with severe acute kidney injury. We describe the study design and discuss aspects of the need for a trial in this patient cohort.
Methods/design
Our plan is to randomize critically ill patients with acute kidney injury to ‘early’ or ‘late’ initiation of renal replacement therapy according to stage 2 and 3 of the KDIGO classification using a specific trial protocol. We plan to guide data collection and analysis using pre-existing definitions and testing. The primary endpoint is overall survival in a 90-day follow-up period. Secondary endpoints include 28-day, 60-day, 90-day and 1-year all-cause mortality, recovery of renal function, ICU and hospital length-of-stay. The primary analysis will be an intention-to-treat analysis; secondary analyses include treated analyses. We will also specify rules for handling data and determining outcome.
Related collections
Most cited references32
- Record: found
- Abstract: found
- Article: not found
Septic acute kidney injury in critically ill patients: clinical characteristics and outcomes.
- Record: found
- Abstract: found
- Article: not found
A comparison of the RIFLE and AKIN criteria for acute kidney injury in critically ill patients.
- Record: found
- Abstract: found
- Article: not found