- Record: found
- Abstract: found
- Article: found
EDD, a Ubiquitin-protein Ligase of the N-end Rule Pathway, Associates with Spindle Assembly Checkpoint Components and Regulates the Mitotic Response to Nocodazole*
Read this article at
Abstract
Background: Mitotic progression is regulated by the spindle assembly checkpoint (SAC) to prevent aneuploidy and chromosome damage.
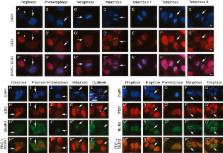
Results: EDD binds to various SAC components, governs the expression levels of key mitosis-associated proteins, and mediates the response to the mitotic spindle poison nocodazole.
Conclusion: EDD contributes to the ability of the SAC to mediate checkpoint arrest.
Significance: EDD may act to maintain genomic integrity.
Abstract
In this work, we identify physical and genetic interactions that implicate E3 identified by differential display (EDD) in promoting spindle assembly checkpoint (SAC) function. During mitosis, the SAC initiates a mitotic checkpoint in response to chromosomes with kinetochores unattached to spindle pole microtubules. Similar to Budding uninhibited by benzimidazoles-related 1 (BUBR1) siRNA, a bona fide SAC component, EDD siRNA abrogated G 2/M accumulation in response to the mitotic destabilizing agent nocodazole. Furthermore, EDD siRNA reduced mitotic cell viability and, in nocodazole-treated cells, increased expression of the promitotic progression protein cell division cycle 20 (CDC20). Copurification studies also identified physical interactions with CDC20, BUBR1, and other components of the SAC. Taken together, these observations highlight the potential role of EDD in regulating mitotic progression and the cellular response to perturbed mitosis.
Related collections
Most cited references39
- Record: found
- Abstract: found
- Article: not found
Aurora B couples chromosome alignment with anaphase by targeting BubR1, Mad2, and Cenp-E to kinetochores
- Record: found
- Abstract: found
- Article: not found
Chromosome segregation errors as a cause of DNA damage and structural chromosome aberrations.
- Record: found
- Abstract: found
- Article: not found