- Record: found
- Abstract: found
- Article: found
Human double negative T cells target lung cancer via ligand-dependent mechanisms that can be enhanced by IL-15
Read this article at
Abstract
Background
The advents of novel immunotherapies have revolutionized the treatment of cancer. Adoptive cellular therapies using chimeric antigen receptor T (CAR-T) cells have achieved remarkable clinical responses in B cell leukemia and lymphoma but the effect on solid tumors including lung cancer is limited. Here we present data on the therapeutic potential of allogeneic CD3 +CD4 −CD8 − double negative T (DNT) cells as a new cellular therapy for the treatment of lung cancer and underlying mechanisms.
Methods
DNTs were enriched and expanded ex vivo from healthy donors and phenotyped by flow cytometry. Functionally, their cytotoxicity was determined against primary and established non-small-cell lung cancer (NSCLC) cell lines in vitro or through in vivo adoptive transfer into xenograft models. Mechanistic analysis was performed using blocking antibodies against various cell surface and soluble markers. Furthermore, the role of IL-15 on DNT function was determined.
Results
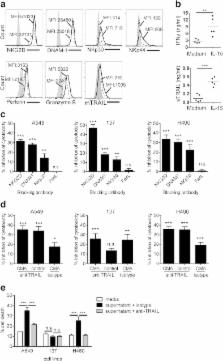
We demonstrated that ex vivo expanded DNTs can effectively lyse various human NSCLC cells in vitro and inhibit tumor growth in xenograft models. Expanded DNTs have a cytotoxic phenotype, as they express NKp30, NKG2D, DNAM-1, membrane TRAIL (mTRAIL), perforin and granzyme B, and secrete IFNγ and soluble TRAIL (sTRAIL). DNT-mediated cytotoxicity was dependent on a combination of tumor-expressed ligands for NKG2D, DNAM-1, NKp30 and/or receptors for TRAIL, which differ among different NSCLC cell lines. Furthermore, stimulation of DNTs with IL-15 increased expression of effector molecules on DNTs, their TRAIL production and cytotoxicity against NSCLC in vitro and in vivo.
Related collections
Most cited references33
- Record: found
- Abstract: found
- Article: not found
Redistribution, hyperproliferation, activation of natural killer cells and CD8 T cells, and cytokine production during first-in-human clinical trial of recombinant human interleukin-15 in patients with cancer.
- Record: found
- Abstract: found
- Article: not found
Regional delivery of mesothelin-targeted CAR T cell therapy generates potent and long-lasting CD4-dependent tumor immunity.
- Record: found
- Abstract: found
- Article: not found