- Record: found
- Abstract: found
- Article: found
Impaired pressure natriuresis and non‐dipping blood pressure in rats with early type 1 diabetes mellitus
Read this article at
Abstract
Key points
-
Type 1 diabetes mellitus increases cardiovascular risk; hypertension amplifies this risk, while pressure natriuresis regulates long‐term blood pressure.
-
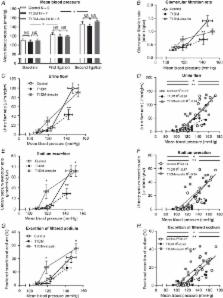
We induced type 1 diabetes in rats by streptozotocin injection and demonstrated a substantial impairment of pressure natriuresis: acute increases in blood pressure did not increase renal medullary blood flow, tubular sodium reabsorption was not downregulated, and proximal tubule sodium reabsorption, measured by lithium clearance, was unaffected.
-
Insulin reduced blood glucose in diabetic rats, and rescued the pressure natriuresis response without influencing lithium clearance, but did not restore medullary blood flow.
-
Radiotelemetry showed that diastolic blood pressure was increased in diabetic rats, and its diurnal variation was reduced.
-
Increases in medullary blood flow and decreases in distal tubule sodium reabsorption that offset acute rises in BP are impaired in early type 1 diabetes, and this impairment could be a target for preventing hypertension in type 1 diabetes.
Abstract
Type 1 diabetes mellitus (T1DM) substantially increases cardiovascular risk, and hypertension amplifies this risk. Blood pressure (BP) and body sodium homeostasis are linked. T1DM patients have increased total exchangeable sodium, correlating directly with BP. Pressure natriuresis is an important physiological regulator of BP. We hypothesised that pressure natriuresis would be impaired, and BP increased, in the early phase of T1DM. Male Sprague‐Dawley rats were injected with streptozotocin (30–45 mg/kg) or citrate vehicle. After 3 weeks, pressure natriuresis was induced by serial arterial ligation. In non‐diabetic controls, this increased fractional excretion of sodium from ∼1% to ∼25% of the filtered load ( P < 0.01); in T1DM rats, the response was significantly blunted, peaking at only ∼3% ( P < 0.01). Mechanistically, normal lithium clearance suggested that distal tubule sodium reabsorption was not downregulated with increased BP in T1DM rats. The pressure dependence of renal medullary perfusion, considered a key factor in the integrated response, was abolished. Insulin therapy rescued the natriuretic response in diabetic rats, restoring normal downregulation of tubular sodium reabsorption when BP was increased. However, the pressure dependence of medullary perfusion was not restored, suggesting persistent vascular dysfunction despite glycaemic control. Radiotelemetry showed that T1DM did not affect systolic BP, but mean diastolic BP was ∼5 mmHg higher than in non‐diabetic controls ( P < 0.01), and normal diurnal variation was reduced. In conclusion, functional impairment of renal sodium and BP homeostasis is an early manifestation of T1DM, preceding hypertension and nephropathy. Early intervention to restore pressure natriuresis in T1DM may complement reductions in cardiovascular risk achieved with glycaemic control.
Key points
-
Type 1 diabetes mellitus increases cardiovascular risk; hypertension amplifies this risk, while pressure natriuresis regulates long‐term blood pressure.
-
We induced type 1 diabetes in rats by streptozotocin injection and demonstrated a substantial impairment of pressure natriuresis: acute increases in blood pressure did not increase renal medullary blood flow, tubular sodium reabsorption was not downregulated, and proximal tubule sodium reabsorption, measured by lithium clearance, was unaffected.
-
Insulin reduced blood glucose in diabetic rats, and rescued the pressure natriuresis response without influencing lithium clearance, but did not restore medullary blood flow.
-
Radiotelemetry showed that diastolic blood pressure was increased in diabetic rats, and its diurnal variation was reduced.
-
Increases in medullary blood flow and decreases in distal tubule sodium reabsorption that offset acute rises in BP are impaired in early type 1 diabetes, and this impairment could be a target for preventing hypertension in type 1 diabetes.
Related collections
Most cited references45
- Record: found
- Abstract: found
- Article: not found
Principles and standards for reporting animal experiments in The Journal of Physiology and Experimental Physiology.
- Record: found
- Abstract: found
- Article: not found
Renal Dysfunction, Rather Than Nonrenal Vascular Dysfunction, Mediates Salt-Induced Hypertension.
- Record: found
- Abstract: found
- Article: not found