- Record: found
- Abstract: found
- Article: found
Free Triiodothyronine Level Correlates with Myocardial Injury and Prognosis in Idiopathic Dilated Cardiomyopathy: Evidence from Cardiac MRI and SPECT/PET Imaging
Read this article at
Abstract
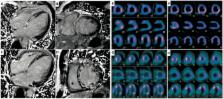
Thyroid dysfunction is associated with poor prognosis in heart failure, but theories of mechanisms are mainly based on animal experiments, not on human level. We aimed to explore the relation between thyroid function and myocardial injuries in idiopathic dilated cardiomyopathy (IDCM) using cardiac magnetic resonance imaging (MRI), single-photon emission computed tomography (SPECT) and positron emission tomography (PET). Myocardial fibrosis was detected by late gadolinium enhancement (LGE) MRI, and myocardial perfusion/metabolism was evaluated by 99mTc-MIBI SPECT / 18F-FDG PET imaging. Across the quartiles of FT3, decreased percentage of segments with LGE and perfusion/metabolism abnormalities were found. As for FT4 and TSH levels, no significant distribution trend of myocardial injuries could be detected. In logistic analysis, FT3 was independently associated with the presence of LGE (OR: 0.140, 95% CI: 0.035–0.567), perfusion abnormalities (OR: 0.172, 95% CI: 0.040–0.738) and metabolism abnormalities (OR: 0.281, 95% CI: 0.081–0.971). After a median follow-up of 46 months, LGE-positive and FT3 < 2.77 pg/mL was identified as the strongest predictor of cardiac events (HR: 8.623, 95% CI: 3.626–16.438). Low FT3 level is associated with myocardial fibrosis and perfusion/metabolism abnormalities in patients with IDCM. The combination of FT3 level and LGE provides useful information for assessing the prognosis of IDCM.
Related collections
Most cited references24
- Record: found
- Abstract: found
- Article: not found
Association of fibrosis with mortality and sudden cardiac death in patients with nonischemic dilated cardiomyopathy.
- Record: found
- Abstract: found
- Article: not found
Subclinical thyroid dysfunction and the risk of heart failure events: an individual participant data analysis from 6 prospective cohorts.
- Record: found
- Abstract: found
- Article: not found