- Record: found
- Abstract: found
- Article: found
Glucose Transporter 1 Promotes the Malignant Phenotype of Non-Small Cell Lung Cancer through Integrin β1/Src/FAK Signaling
Read this article at
Abstract
Background: Glucose transporter 1 (GLUT1) is the main factor of Warburg effect, which is associated with poor prognosis in many tumors. However, the underlying molecular mechanism of GLUT1 in the progression of non-small cell lung cancer (NSCLC) is unclear.
Methods: We used quantitative real-time PCR to detect GLUT1 mRNA expression in bronchial brushing samples and performed Western Blot and biological behavior testing to check the effect of GLUT1 on NSCLC cell proliferation, migration, invasion and apoptosis.
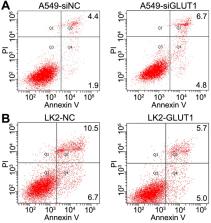
Results: We found that the C(t) normalized value of GLUT1 in malignant bronchial brushing samples was significantly higher than that in benign samples ( P<0.05). GLUT1 significantly increased the expressions of cyclin A, cyclin D1, cyclin E, cyclin dependent kinase 2 (CDK2), CDK4, CDK6 and matrix metalloproteinase 2 (MMP2), but decreased the expressions of p53 and p130 in NSCLC cells. The biological behavior testing indicated that GLUT1 enhanced NSCLC cell proliferation, invasion and migration but inhibited cell apoptosis. In addition, GLUT1 upregulated the expression of integrin β1 and promoted the phosphorylation of focal adhesion kinase (FAK, phosphorylation at Tyr576/577) and Src (Src phosphorylation at Tyr530). siRNA knock down of integrin β1 expression suppressed GLUT1 induced NSCLC cell biological behavior, as well as the phosphorylation of FAK and Src.
Conclusion: Taken together, our data confirms that GLUT1 promotes the malignant phenotype of NSCLC through integrin β1/Src/FAK signaling, which provides a new therapeutic target for the treatment and research of lung cancer.
Related collections
Most cited references46
- Record: found
- Abstract: found
- Article: not found
Genetic and cell biological analysis of integrin outside-in signaling.
- Record: found
- Abstract: found
- Article: not found
Autophosphorylation of the focal adhesion kinase, pp125FAK, directs SH2-dependent binding of pp60src.
- Record: found
- Abstract: found
- Article: not found