- Record: found
- Abstract: found
- Article: not found
A palladium-catalysed enolate alkylation cascade for the formation of adjacent quaternary and tertiary stereocentres
Read this article at
Abstract
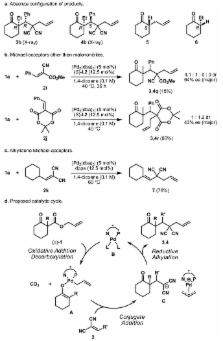
The catalytic enantioselective synthesis of densely functionalised organic molecules containing all-carbon quaternary stereocentres is a challenge to modern chemical methodology research. The catalytically controlled asymmetric α-alkylation of ketones represents another difficult task and has been of major interest to our and other research groups in the past. We now report a palladium-catalyzed enantioselective process that addresses both problems at once and allows the installation of vicinal all-carbon quaternary and tertiary stereocentres at the α-carbon of a ketone in a single step. This multiple bond forming process is carried out on readily available β-ketoester starting materials and proceeds via conjugate addition of an in situ-generated palladium enolate to activated Michael acceptors. In other words, the CO 2-moiety of the substrate is displaced by a C-C fragment in an asymmetric cut-and-paste reaction. The products are obtained in high yield, diastereomeric ratio, and enantiomeric excess.
Related collections
Most cited references44
- Record: found
- Abstract: not found
- Article: not found
Catalytic Enantioselective Construction of All-Carbon Quaternary Stereocenters
- Record: found
- Abstract: found
- Article: not found
