- Record: found
- Abstract: found
- Article: found
Effects of Ethanol Extracts from Grateloupia elliptica, a Red Seaweed, and Its Chlorophyll Derivative on 3T3-L1 Adipocytes: Suppression of Lipid Accumulation through Downregulation of Adipogenic Protein Expression
Read this article at
Abstract
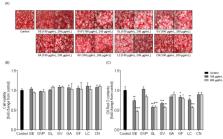
Grateloupia elliptica ( G. elliptica) is a red seaweed with antioxidant, antidiabetic, anticancer, anti-inflammatory, and anticoagulant activities. However, the anti-obesity activity of G. elliptica has not been fully investigated. Therefore, the effect of G. elliptica ethanol extract on the suppression of intracellular lipid accumulation in 3T3-L1 cells by Oil Red O staining (ORO) was evaluated. Among the eight red seaweeds tested, G. elliptica 60% ethanol extract (GEE) exhibited the highest inhibition of lipid accumulation. GEE was the only extract to successfully suppress lipid accumulation among ethanol extracts from eight red seaweeds. In this study, we successfully isolated chlorophyll derivative (CD) from the ethyl acetate fraction (EA) of GEE by high-performance liquid chromatography and evaluated their inhibitory effect on intracellular lipid accumulation in 3T3-L1 adipocytes. CD significantly suppressed intracellular lipid accumulation. In addition, CD suppressed adipogenic protein expression such as sterol regulatory element-binding protein-1 (SREBP-1), peroxisome proliferator-activated receptor-γ (PPAR-γ), CCAAT/enhancer-binding protein-α (C/EBP-α), and fatty acid binding protein 4 (FABP4). Taken together, our results indicate that CD from GEE inhibits lipid accumulation by suppressing adipogenesis via the downregulation of adipogenic protein expressions in the differentiated adipocytes. Therefore, chlorophyll from G. elliptica has a beneficial effect on lipid metabolism and it could be utilized as a potential therapeutic agent for preventing obesity.
Related collections
Most cited references76
- Record: found
- Abstract: found
- Article: not found
Combined analysis of oligonucleotide microarray data from transgenic and knockout mice identifies direct SREBP target genes.
- Record: found
- Abstract: not found
- Article: not found