- Record: found
- Abstract: found
- Article: found
Kampo medicines, Rokumigan, Hachimijiogan, and Goshajinkigan, significantly inhibit glucagon-induced CREB activation
Read this article at
Abstract
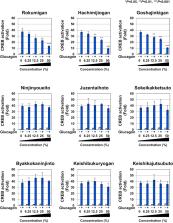
The pathophysiology of type 2 diabetes mellitus (T2DM) is characterized by not only insulin resistance, but also the abnormal regulation of glucagon secretion, suggesting that antagonizing the glucagon-induced signaling pathway has therapeutic potential in the treatment of T2DM. Although various Kampo medicines (traditional herbal medicines) are often utilized to ameliorate the symptoms of T2DM, their effects on glucagon signaling have not yet been clarified. In the present study, we examined the effects of nine types of representative Kampo formulations prescribed for T2DM on glucagon-induced CREB activation in HEK293T cells stably expressing glucagon receptor (Gcgr) and a hepatic cell line HepG2. Among these Kampo medicines, Rokumigan, Hachimijiogan, and Goshajinkigan significantly suppressed the glucagon-induced transactivation of the cAMP-responsive element (CRE)-binding protein (CREB) by inhibiting its interaction with CREB-binding protein (CBP), which led to a reduction in the expression of phosphoenolpyruvate carboxykinase (PEPCK) mRNA. Furthermore, among the crude drugs commonly contained in these three Kampo medicines, Rehmannia Root (Jio), Moutan Bark (Botampi), and Cornus Fruit (Shanzhuyu) exerted inhibitory effects on glucagon-induced CREB activation. Collectively, the present results provide a novel mechanism, the inhibition of glucagon signaling, by which Rokumigan, Hachimijiogan, and Goshajinkigan improve the symptoms of T2DM.
Abstract
Cell biology; Pharmaceutical science; Biochemistry; Molecular biology; Health sciences; Pharmacology; Type 2 diabetes mellitus; Kampo medicines; Glucagon; CREB; PEPCK
Related collections
Most cited references29
- Record: found
- Abstract: found
- Article: not found
Cyclic AMP stimulates somatostatin gene transcription by phosphorylation of CREB at serine 133.
- Record: found
- Abstract: found
- Article: not found
Phosphorylated CREB binds specifically to the nuclear protein CBP.
- Record: found
- Abstract: found
- Article: not found