- Record: found
- Abstract: found
- Article: found
Impact of Pneumococcal Conjugate Vaccination on Otitis Media: A Systematic Review
Read this article at
Abstract
Reduced rates of consultations for otitis media after introduction of pneumococcal conjugate vaccines (PCVs) have been overinterpreted. This systematic review suggests that PCV is only somewhat modestly effective against all-cause otitis media.
Abstract
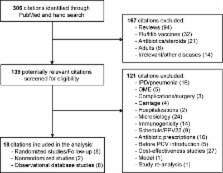
Acute otitis media (AOM) is a leading cause of visits to physicians and of antibiotic prescriptions for young children. We systematically reviewed studies on all-cause AOM episodes and physician visits in which impact was attributed to pneumococcal conjugate vaccines, either as efficacy or effectiveness. Of 18 relevant publications found, most used the 7-valent pneumococcal conjugate vaccine (7vCRM). The efficacy of 7vCRM against all-cause AOM episodes or visits was 0%–9% in randomized trials and 17%–23% in nonrandomized trials. In observational database studies, physician visits for AOM were already declining in the 3–5 years before 7vCRM introduction (mean change, −15%; range, +14% to −24%) and continued to decline afterward (mean, −19%; range, +7% to −48%). This vaccine provides some protection against OM, but other factors have also contributed to the recent decline in OM incidence. Future effectiveness studies should thus use better-controlled methods to estimate the true impact of vaccination on AOM.
Related collections
Most cited references57
- Record: found
- Abstract: found
- Article: not found
Herd immunity and serotype replacement 4 years after seven-valent pneumococcal conjugate vaccination in England and Wales: an observational cohort study.
- Record: found
- Abstract: found
- Article: not found
Efficacy of a pneumococcal conjugate vaccine against acute otitis media.
- Record: found
- Abstract: found
- Article: not found