- Record: found
- Abstract: found
- Article: found
Digital health applications from a government-regulated directory of reimbursable health apps in Germany—a systematic review for evidence and bias
Read this article at
Abstract
Background
The Digital Healthcare Act, passed in November 2019, authorizes healthcare providers in Germany to prescribe digital health applications (DiGA) to patients covered by statutory health insurance. If DiGA meet specific efficacy requirements, they may be listed in a special directory maintained by the German Federal Institute for Drugs and Medical Devices. Due to the lack of well-founded app evaluation tools, the objectives were to assess (I) the evidence quality situation for DiGA in the literature and (II) how DiGA manufacturers deal with this issue, as reflected by the apps available in the aforementioned directory.
Methods
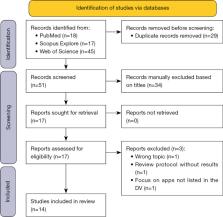
A systematic review of the literature on DiGA using PubMed, Scopus, and Web of Science was started on February 4, 2023. Papers addressing the evidence for applications listed in the directory were included, while duplicates and mere study protocols not reporting on data were removed. The remaining publications were used to assess the quality of the evidence or potential gaps in this regard. Results were aggregated in tabular form.
Results
The review identified fourteen relevant publications. Six studies suggested inadequate scientific evidence, five mentioned shortcomings of tools for validating DiGA-related evidence, and four publications described a high potential for bias, potentially influencing the validity of the results. Concerns about limited external generalizability were also raised.
Related collections
Most cited references22

- Record: found
- Abstract: found
- Article: found
The PRISMA 2020 statement: an updated guideline for reporting systematic reviews

- Record: found
- Abstract: found
- Article: found
Health App Use Among US Mobile Phone Owners: A National Survey

- Record: found
- Abstract: found
- Article: found