- Record: found
- Abstract: found
- Article: found
Optical Coherence Tomography Angiography Vessel Density in Healthy, Glaucoma Suspect, and Glaucoma Eyes
Read this article at
Abstract
Purpose
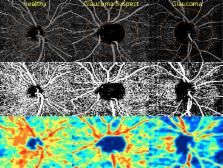
The purpose of this study was to compare retinal nerve fiber layer (RNFL) thickness and optical coherence tomography angiography (OCT-A) retinal vasculature measurements in healthy, glaucoma suspect, and glaucoma patients.
Methods
Two hundred sixty-one eyes of 164 healthy, glaucoma suspect, and open-angle glaucoma (OAG) participants from the Diagnostic Innovations in Glaucoma Study with good quality OCT-A images were included. Retinal vasculature information was summarized as a vessel density map and as vessel density (%), which is the proportion of flowing vessel area over the total area evaluated. Two vessel density measurements extracted from the RNFL were analyzed: (1) circumpapillary vessel density (cpVD) measured in a 750-μm-wide elliptical annulus around the disc and (2) whole image vessel density (wiVD) measured over the entire image. Areas under the receiver operating characteristic curves (AUROC) were used to evaluate diagnostic accuracy.
Results
Age-adjusted mean vessel density was significantly lower in OAG eyes compared with glaucoma suspects and healthy eyes. (cpVD: 55.1 ± 7%, 60.3 ± 5%, and 64.2 ± 3%, respectively; P < 0.001; and wiVD: 46.2 ± 6%, 51.3 ± 5%, and 56.6 ± 3%, respectively; P < 0.001). For differentiating between glaucoma and healthy eyes, the age-adjusted AUROC was highest for wiVD (0.94), followed by RNFL thickness (0.92) and cpVD (0.83). The AUROCs for differentiating between healthy and glaucoma suspect eyes were highest for wiVD (0.70), followed by cpVD (0.65) and RNFL thickness (0.65).
Related collections
Most cited references50

- Record: found
- Abstract: found
- Article: found
Split-spectrum amplitude-decorrelation angiography with optical coherence tomography
- Record: found
- Abstract: found
- Article: not found
Optical coherence tomography angiography of optic disc perfusion in glaucoma.
- Record: found
- Abstract: found
- Article: not found
Optical Coherence Tomography Angiography of the Peripapillary Retina in Glaucoma.
Author and article information
Comments
Comment on this article
 Smart Citations
Smart CitationsSee how this article has been cited at scite.ai
scite shows how a scientific paper has been cited by providing the context of the citation, a classification describing whether it supports, mentions, or contrasts the cited claim, and a label indicating in which section the citation was made.