- Record: found
- Abstract: found
- Article: found
Low-intensity Pulsed Ultrasound regulates alveolar bone homeostasis in experimental Periodontitis by diminishing Oxidative Stress
Read this article at
Abstract
Periodontitis is a widespread oral disease that results in the loss of alveolar bone. Low-intensity pulsed ultrasound (LIPUS), which is a new therapeutic option, promotes alveolar bone regeneration in periodontal bone injury models. This study investigated the protective effect of LIPUS on oxidative stress in periodontitis and the mechanism underlying this process.
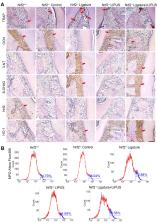
Methods: An experimental periodontitis model was induced by administering a ligature . Immunohistochemistry was performed to detect the expression levels of oxidative stress, osteogenic, and osteoclastogenic markers in vivo. Cell viability and osteogenic differentiation were analyzed using the Cell Counting Kit-8, alkaline phosphatase, and Alizarin Red staining assays. A reactive oxygen species assay kit, lipid peroxidation MDA assay kit, and western blotting were used to determine oxidative stress status in vitro. To verify the role of nuclear factor erythroid 2-related factor 2 (Nrf2), an oxidative regulator, during LIPUS treatment, the siRNA technique and Nrf2 -/- mice were used. The PI3K/Akt inhibitor LY294002 was utilized to identify the effects of the PI3K-Akt/Nrf2 signaling pathway.
Results: Alveolar bone resorption, which was experimentally induced by periodontitis in vivo, was alleviated by LIPUS via activation of Nrf2. Oxidative stress, induced via H 2O 2 treatment in vitro, inhibited cell viability and suppressed osteogenic differentiation. These effects were also alleviated by LIPUS treatment via Nrf2 activation. Nrf2 silencing blocked the antioxidant effect of LIPUS by diminishing heme oxygenase-1 expression. Nrf2 -/- mice were susceptible to ligature-induced periodontitis, and the protective effect of LIPUS on alveolar bone dysfunction was weaker in these mice. Activation of Nrf2 by LIPUS was accompanied by activation of the PI3K/Akt pathway. The oxidative defense function of LIPUS was inhibited by exposure to LY294002 in vitro.
Conclusions: These results demonstrated that LIPUS regulates alveolar bone homeostasis in periodontitis by attenuating oxidative stress via the regulation of PI3K-Akt/Nrf2 signaling. Thus, Nrf2 plays a pivotal role in the protective effect exerted by LIPUS against ligature-induced experimental periodontitis.
Related collections
Most cited references69
- Record: found
- Abstract: found
- Article: not found
The Nrf2-antioxidant response element signaling pathway and its activation by oxidative stress.
- Record: found
- Abstract: found
- Article: not found
Periodontitis: a polymicrobial disruption of host homeostasis.
- Record: found
- Abstract: found
- Article: not found