- Record: found
- Abstract: found
- Article: found
Concurrent once-daily versus twice-daily chemoradiotherapy in patients with limited-stage small-cell lung cancer (CONVERT): an open-label, phase 3, randomised, superiority trial
Read this article at
Summary
Background
Concurrent chemoradiotherapy is the standard of care in limited-stage small-cell lung cancer, but the optimal radiotherapy schedule and dose remains controversial. The aim of this study was to establish a standard chemoradiotherapy treatment regimen in limited-stage small-cell lung cancer.
Methods
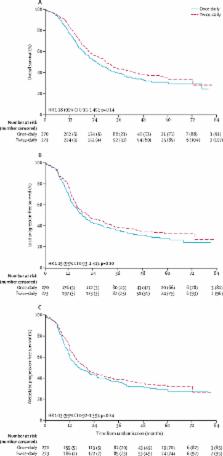
The CONVERT trial was an open-label, phase 3, randomised superiority trial. We enrolled adult patients (aged ≥18 years) who had cytologically or histologically confirmed limited-stage small-cell lung cancer, Eastern Cooperative Oncology Group performance status of 0–2, and adequate pulmonary function. Patients were recruited from 73 centres in eight countries. Patients were randomly assigned to receive either 45 Gy radiotherapy in 30 twice-daily fractions of 1·5 Gy over 19 days, or 66 Gy in 33 once-daily fractions of 2 Gy over 45 days, starting on day 22 after commencing cisplatin–etoposide chemotherapy (given as four to six cycles every 3 weeks in both groups). The allocation method used was minimisation with a random element, stratified by institution, planned number of chemotherapy cycles, and performance status. Treatment group assignments were not masked. The primary endpoint was overall survival, defined as time from randomisation until death from any cause, analysed by modified intention-to-treat. A 12% higher overall survival at 2 years in the once-daily group versus the twice-daily group was considered to be clinically significant to show superiority of the once-daily regimen. The study is registered with ClinicalTrials.gov (NCT00433563) and is currently in follow-up.
Findings
Between April 7, 2008, and Nov 29, 2013, 547 patients were enrolled and randomly assigned to receive twice-daily concurrent chemoradiotherapy (274 patients) or once-daily concurrent chemoradiotherapy (273 patients). Four patients (one in the twice-daily group and three in the once-daily group) did not return their case report forms and were lost to follow-up; these patients were not included in our analyses. At a median follow-up of 45 months (IQR 35–58), median overall survival was 30 months (95% CI 24–34) in the twice-daily group versus 25 months (21–31) in the once-daily group (hazard ratio for death in the once daily group 1·18 [95% CI 0·95–1·45]; p=0·14). 2-year overall survival was 56% (95% CI 50–62) in the twice-daily group and 51% (45–57) in the once-daily group (absolute difference between the treatment groups 5·3% [95% CI −3·2% to 13·7%]). The most common grade 3–4 adverse event in patients evaluated for chemotherapy toxicity was neutropenia (197 [74%] of 266 patients in the twice-daily group vs 170 [65%] of 263 in the once-daily group). Most toxicities were similar between the groups, except there was significantly more grade 4 neutropenia with twice-daily radiotherapy (129 [49%] vs 101 [38%]; p=0·05). In patients assessed for radiotherapy toxicity, was no difference in grade 3–4 oesophagitis between the groups (47 [19%] of 254 patients in the twice-daily group vs 47 [19%] of 246 in the once-daily group; p=0·85) and grade 3–4 radiation pneumonitis (4 [3%] of 254 vs 4 [2%] of 246; p=0·70). 11 patients died from treatment-related causes (three in the twice-daily group and eight in the once-daily group).
Interpretation
Survival outcomes did not differ between twice-daily and once-daily concurrent chemoradiotherapy in patients with limited-stage small-cell lung cancer, and toxicity was similar and lower than expected with both regimens. Since the trial was designed to show superiority of once-daily radiotherapy and was not powered to show equivalence, the implication is that twice-daily radiotherapy should continue to be considered the standard of care in this setting.
Related collections
Most cited references17
- Record: found
- Abstract: found
- Article: not found
A meta-analysis of thoracic radiotherapy for small-cell lung cancer.
- Record: found
- Abstract: found
- Article: not found
Treatment of small cell lung cancer: Diagnosis and management of lung cancer, 3rd ed: American College of Chest Physicians evidence-based clinical practice guidelines.
- Record: found
- Abstract: found
- Article: not found