- Record: found
- Abstract: found
- Article: found
Capillary Networks for Bio-Artificial Three-Dimensional Tissues Fabricated Using Cell Sheet Based Tissue Engineering
Read this article at
Abstract
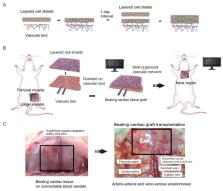
One of the most important challenges facing researchers in the field of regenerative medicine is to develop methods to introduce vascular networks into bioengineered tissues. Although cell scaffolds that slowly release angiogenic factors can promote post-transplantation angiogenesis, they cannot be used to construct thick tissues because of the time required for sufficient vascular network formation. Recently, the co-culture of graft tissue with vascular cells before transplantation has attracted attention as a way of promoting capillary angiogenesis. Although the co-cultured vascular cells can directly contribute to blood vessel formation within the tissue, a key objective that needs to be met is the construction of a continuous circulatory structure. Previously described strategies to reconstruct blood vessels include the culture of endothelial cells in a scaffold that contains microchannels or within the original vascular framework after decellularization of an entire organ. The technique, as developed by authors, involves the progressive stacking of three-layered cell sheets onto a vascular bed to induce the formation of a capillary network within the cell sheets. This approach enables the construction of thick, functional tissue of high cell density that can be transplanted by anastomosing its artery and vein (provided by the vascular bed) with host blood vessels.
Related collections
Most cited references36
- Record: found
- Abstract: found
- Article: not found
Perfusion-decellularized matrix: using nature's platform to engineer a bioartificial heart.
- Record: found
- Abstract: found
- Article: not found