- Record: found
- Abstract: found
- Article: found
Cell–Cell Interaction Proteins (Gap Junctions, Tight Junctions, and Desmosomes) and Water Transporter Aquaporin 4 in Meningothelial Cells of the Human Optic Nerve
Read this article at
Abstract
Purpose
Meningothelial cells (MECs) play a central role in the maintenance of cerebrospinal fluid (CSF) homeostasis and in physiological and pathophysiological processes within the subarachnoid space (SAS) linking them to optic nerve (ON) pathologies. Still, not much is known about their structural properties that might enable MECs to perform specific functions within the ON microenvironment.
Methods
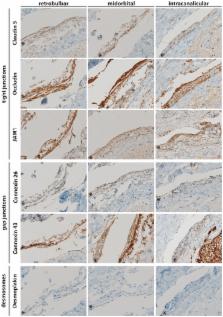
For closer characterization of the structural properties of the human MEC layer in the arachnoid, we performed immunohistological analyses to evaluate the presence of cell–cell interaction markers, namely, markers for tight junctions (JAM1, Occludin, and Claudin 5), gap junctions (Connexin 26 and 43), and desmosomes (Desmoplakin) as well as for water channel marker aquaporin 4 (AQP4) in retrobulbar, midorbital, and intracanalicular human ON sections.
Results
MECs displayed immunopositivity for markers of tight junctions (JAM1, Occludin, and Claudin 5) and gap junctions (Connexin 26 and 43) as well as for AQP4 water channels. However, no immunopositivity was found for Desmoplakin.
Conclusion
MECs are connected via tight junctions and gap junctions, and they possess AQP4 water channels. The presence of these proteins emphasizes the important function of MECs within the ON microenvironment as part of the meningeal barrier. Beyond this barrier function, the expression of these proteins by MECs supports a broader role of these cells in signal transduction and CSF clearance pathways within the ON microenvironment.
Related collections
Most cited references16
- Record: found
- Abstract: found
- Article: not found
Endothelial cell-to-cell junctions: molecular organization and role in vascular homeostasis.
- Record: found
- Abstract: found
- Article: not found
Architecture of arachnoid trabeculae, pillars, and septa in the subarachnoid space of the human optic nerve: anatomy and clinical considerations.
- Record: found
- Abstract: found
- Article: not found