- Record: found
- Abstract: found
- Article: found
Monocytes Loaded with Indocyanine Green as Active Homing Contrast Agents Permit Optical Differentiation of Infectious and Non-Infectious Inflammation
Read this article at
Abstract
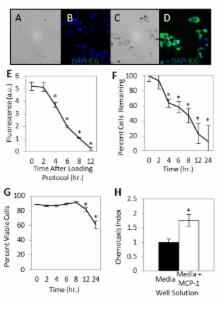
Distinguishing cutaneous infection from sterile inflammation is a diagnostic challenge and currently relies upon subjective interpretation of clinical parameters, microbiological data, and nonspecific imaging. Assessing characteristic variations in leukocytic infiltration may provide more specific information. In this study, we demonstrate that homing of systemically administered monocytes tagged using indocyanine green (ICG), an FDA-approved near infrared dye, may be assessed non-invasively using clinically-applicable laser angiography systems to investigate cutaneous inflammatory processes. RAW 264.7 mouse monocytes co-incubated with ICG fluoresce brightly in the near infrared range. In vitro, the loaded cells retained the ability to chemotax toward monocyte chemotactic protein-1. Following intravascular injection of loaded cells into BALB/c mice with induced sterile inflammation (Complete Freund’s Adjuvant inoculation) or infection (Group A Streptococcus inoculation) of the hind limb, non-invasive whole animal imaging revealed local fluorescence at the inoculation site. There was significantly higher fluorescence of the inoculation site in the infection model than in the inflammation model as early as 2 hours after injection (p<0.05). Microscopic examination of bacterial inoculation site tissue revealed points of near infrared fluorescence, suggesting the presence of ICG-loaded cells. Development of a non-invasive technique to rapidly image inflammatory states without radiation may lead to new tools to distinguish infectious conditions from sterile inflammatory conditions at the bedside.
Related collections
Most cited references23
- Record: found
- Abstract: found
- Article: not found
Trends in US Hospital Admissions for Skin and Soft Tissue Infections
- Record: found
- Abstract: found
- Article: not found
Comparative analysis of USA300 virulence determinants in a rabbit model of skin and soft tissue infection.

- Record: found
- Abstract: found
- Article: found