- Record: found
- Abstract: found
- Article: not found
Cloning and Characterization of a Specific Receptor for the Novel CC Chemokine MIP-3α from Lung Dendritic Cells
Read this article at
Abstract
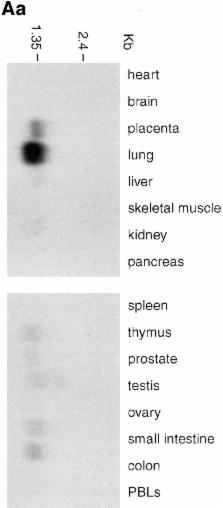
Dendritic cells are potent antigen-presenting cells involved in the initiation of immune responses. The trafficking of these cells to tissues and lymph nodes is mediated by members of the chemokine family. Recently, a novel CC chemokine known as MIP-3α or liver and activation-regulated chemokine has been identified from the EMBL/GenBank/DDBJ expressed sequence tag database. In the present study, we have shown that the messenger RNA for MIP-3α is expressed predominantly in inflamed and mucosal tissues. MIP-3α produced either synthetically or by human embryonic kidney 293 cells is chemotactic for CD34 +-derived dendritic cells and T cells, but is inactive on monocytes and neutrophils. MIP-3α was unable to displace the binding of specific CC or CXC chemokines to stable cell lines expressing their respective high affinity receptors, namely CCR1–5 and CXCR1 and CXCR2, suggesting that MIP-3α acts through a novel CC chemokine receptor. Therefore, we used degenerate oligonucleotide-based reverse transcriptase PCR to identify candidate MIP-3α receptors in lung dendritic cells. Our results show that the orphan receptor known as GCY-4, CKRL-3, or STRL-22 is a specific receptor for MIP-3α, and that its activation leads to pertussis toxin–sensitive and phospholipase C–dependent intracellular Ca 2+ mobilization when it is expressed in HEK 293 cells.
Related collections
Most cited references40
- Record: found
- Abstract: found
- Article: not found
Chemokine receptor specific for IP10 and mig: structure, function, and expression in activated T-lymphocytes
- Record: found
- Abstract: found
- Article: not found
Molecular cloning and functional expression of two monocyte chemoattractant protein 1 receptors reveals alternative splicing of the carboxyl-terminal tails.
- Record: found
- Abstract: found
- Article: not found