- Record: found
- Abstract: found
- Article: found
Clinical performance of an antibody-free assay for plasma Aβ42/Aβ40 to detect early alterations of Alzheimer’s disease in individuals with subjective cognitive decline
Read this article at
Abstract
Background
Accessible and cost-effective diagnostic tools are urgently needed to accurately quantify blood biomarkers to support early diagnosis of Alzheimer’s disease (AD). In this study, we investigated the ability of plasma amyloid-beta (Aβ)42/Aβ40 ratio measured by an antibody-free mass-spectrometric (MS) method, ABtest-MS, to detect early pathological changes of AD.
Methods
This cohort study included data from the baseline and 2-year follow-up visits from the Fundació ACE Healthy Brain Initiative (FACEHBI) study. Plasma Aβ42/Aβ40 was measured with ABtest-MS and compared to 18F-Florbetaben PET as the reference standard (cutoff for early amyloid deposition of 13.5 centiloids). Cross-validation was performed in an independent DPUK-Korean cohort. Additionally, associations of plasma Aβ42/Aβ40 with episodic memory performance and brain atrophy were assessed.
Results
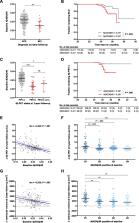
The FACEHBI cohort at baseline included 200 healthy individuals with subjective cognitive decline (SCD), of which 36 (18%) were Aβ-PET positive. Plasma Aβ42/Aβ40 levels were significantly lower in Aβ-PET positive individuals (median [interquartile range, IQR], 0.215 [0.203–0.236]) versus Aβ-PET negative subjects (median [IQR], 0.261 [0.244–0.279]) ( P < .001). Plasma Aβ42/Aβ40 was significantly correlated with Aβ-PET levels (rho = −0.390; P < .001) and identified Aβ-PET status with an area under the receiver operating characteristic curve (AUC) of 0.87 (95% confidence interval [CI], 0.80–0.93). A cutoff for the Aβ42/Aβ40 ratio of 0.241 (maximum Youden index) yielded a sensitivity of 86.1% and a specificity of 80.5%. These findings were cross-validated in an independent DPUK-Korean cohort (AUC 0.86 [95% CI 0.77–0.95]). Lower plasma Aβ42/Aβ40 ratio was associated with worse episodic memory performance and increased brain atrophy. Plasma Aβ42/Aβ40 at baseline predicted clinical conversion to mild cognitive impairment and longitudinal changes in amyloid deposition and brain atrophy at 2-year follow-up.
Conclusions
This study suggests that plasma Aβ42/Aβ40, as determined by this MS-based assay, has potential value as an accurate and cost-effective tool to identify individuals in the earliest stages of AD, supporting its implementation in clinical trials, preventative strategies and clinical practice.
Related collections
Most cited references48
- Record: found
- Abstract: found
- Article: not found
High performance plasma amyloid-β biomarkers for Alzheimer’s disease
- Record: found
- Abstract: found
- Article: found
Blood phosphorylated tau 181 as a biomarker for Alzheimer's disease: a diagnostic performance and prediction modelling study using data from four prospective cohorts
- Record: found
- Abstract: found
- Article: found