- Record: found
- Abstract: found
- Article: not found
Supramolecular Self-Assembly To Control Structural and Biological Properties of Multicomponent Hydrogels
Read this article at
Abstract

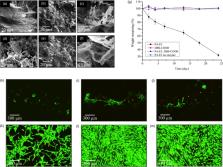
Self-assembled nanofibers are ubiquitous in nature and serve as inspiration for the design of supramolecular hydrogels. A multicomponent approach offers the possibility of enhancing the tunability and functionality of this class of materials. We report on the synergistic multicomponent self-assembly involving a peptide amphiphile (PA) and a 1,3:2,4-dibenzylidene- d-sorbitol (DBS) gelator to generate hydrogels with tunable nanoscale morphology, improved stiffness, enhanced self-healing, and stability to enzymatic degradation. Using induced circular dichroism of Thioflavin T (ThT), electron microscopy, small-angle neutron scattering, and molecular dynamics approaches, we confirm that the PA undergoes self-sorting, while the DBS gelator acts as an additive modifier for the PA nanofibers. The supramolecular interactions between the PA and DBS gelators result in improved bulk properties and cytocompatibility of the two-component hydrogels as compared to those of the single-component systems. The tunable mechanical properties, self-healing ability, resistance to proteolysis, and biocompatibility of the hydrogels suggest future opportunities for the hydrogels as scaffolds for tissue engineering and drug delivery vehicles.
Related collections
Most cited references73
- Record: found
- Abstract: found
- Article: not found
Supramolecular Hydrogelators and Hydrogels: From Soft Matter to Molecular Biomaterials
- Record: found
- Abstract: found
- Article: not found
Supramolecular gels formed from multi-component low molecular weight species.
- Record: found
- Abstract: found
- Article: not found
