- Record: found
- Abstract: found
- Article: not found
Efficacy of nirsevimab against respiratory syncytial virus lower respiratory tract infections in preterm and term infants, and pharmacokinetic extrapolation to infants with congenital heart disease and chronic lung disease: a pooled analysis of randomised controlled trials
Read this article at
Abstract
Background
In a phase 2b trial and the phase 3 MELODY trial, nirsevimab, an extended half-life, monoclonal antibody against respiratory syncytial virus (RSV), protected healthy infants born preterm or at full term against medically attended RSV lower respiratory tract infection (LRTI). In the MEDLEY phase 2–3 trial in infants at higher risk for severe RSV infection, nirsevimab showed a similar safety profile to that of palivizumab. The aim of the current analysis was to assess the efficacy of nirsevimab using a weight-banded dosing regimen in infants born between 29 weeks gestational age and full term.
Methods
Infants enrolled in the phase 2b and MELODY trials were randomised (2:1) to receive a single intramuscular injection of nirsevimab (infants weighing <5 kg received 50 mg; those weighing ≥5 kg received 100 mg) or placebo before the RSV season. Infants in MEDLEY were randomised (2:1) to receive one dose of nirsevimab (infants weighing <5 kg received 50 mg; those weighing ≥5 kg received 100 mg) followed by four monthly placebo doses, or five once-a-month intramuscular doses of palivizumab. We report a prespecified pooled efficacy analysis assessing the weight-banded dosing regimen proposed on the basis of the phase 2b and MELODY trials, in addition to extrapolated efficacy in infants with chronic lung disease, congenital heart disease, or extreme preterm birth (<29 weeks' gestational age) based on pharmacokinetic data from the phase 2–3 MEDLEY safety trial. For the pooled efficacy analysis, the primary endpoint was incidence of medically attended RSV LRTI through 150 days post-dose. The secondary efficacy endpoint was number of admissions to hospital for medically attended RSV LRTI. The incidence of very severe RSV LRTI was an exploratory endpoint, defined as cases of hospital admission for medically attended RSV LRTI that required supplemental oxygen or intravenous fluids. We also did a prespecified exploratory analysis of medically attended LRTI of any cause (in the investigator's judgement) and hospital admission for respiratory illness of any cause (defined as any upper respiratory tract infection or LRTI leading to hospital admission). Post hoc exploratory analyses of outpatient visits and antibiotic use were also done. Nirsevimab serum concentrations in MEDLEY were assessed using population pharmacokinetic methods and the pooled data from the phase 2b and MELODY trials. An exposure target was defined on the basis of an exposure–response analysis. To successfully demonstrate extrapolation, more than 80% of infants in MEDLEY had to achieve serum nirsevimab exposures at or above the predicted efficacious target.
Findings
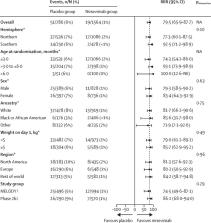
Overall, 2350 infants (1564 in the nirsevimab group and 786 in the placebo group) in the phase 2b and MELODY trials were included in the pooled analysis. Nirsevimab showed efficacy versus placebo with respect to the primary endpoint of medically attended RSV LRTI (19 [1%] nirsevimab recipients vs 51 [6%] placebo recipients; relative risk reduction [RRR] 79·5% [95% CI 65·9–87·7]). Consistent efficacy was shown for additional endpoints of RSV LRTI hospital admission (nine [1%] nirsevimab recipients vs 21 [3%] placebo recipients; 77·3% [50·3–89·7]) and very severe RSV (five [<1%] vs 18 [2%]; 86·0% [62·5–94·8]). Nirsevimab recipients had fewer hospital admissions for any-cause respiratory illness (RRR 43·8% [18·8–61·1]), any-cause medically attended LRTI (35·4% [21·5–46·9]), LRTI outpatient visits (41·9% [25·7–54·6]), and antibiotic prescriptions (23·6% [3·8–39·3]). Among infants with chronic lung disease, congenital heart disease, or extreme preterm birth in MEDLEY, nirsevimab serum exposures were similar to those found in the pooled data; exposures were above the target in more than 80% of the overall MEDLEY trial population (94%), including infants with chronic lung disease (94%) or congenital heart disease (80%) and those born extremely preterm (94%).
Interpretation
A single dose of nirsevimab protected healthy infants born at term or preterm from medically attended RSV LRTI, associated hospital admission, and severe RSV. Pharmacokinetic data support efficacy extrapolation to infants with chronic lung disease, congenital heart disease, or extreme prematurity. Together, these data suggest that nirsevimab has the potential to change the landscape of infant RSV disease by reducing a major cause of infant morbidity and the consequent burden on caregivers, clinicians, and health-care providers.
Related collections
Most cited references31
- Record: found
- Abstract: found
- Article: not found
A modified poisson regression approach to prospective studies with binary data.

- Record: found
- Abstract: found
- Article: not found
