- Record: found
- Abstract: found
- Article: found
Genome editing for horticultural crop improvement
Read this article at
Abstract
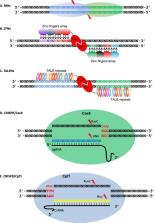
Horticultural crops provide humans with many valuable products. The improvement of the yield and quality of horticultural crops has been receiving increasing research attention. Given the development and advantages of genome-editing technologies, research that uses genome editing to improve horticultural crops has substantially increased in recent years. Here, we briefly review the different genome-editing systems used in horticultural research with a focus on clustered regularly interspaced palindromic repeats (CRISPR)/CRISPR-associated 9 (Cas9)-mediated genome editing. We also summarize recent progress in the application of genome editing for horticultural crop improvement. The combination of rapidly advancing genome-editing technology with breeding will greatly increase horticultural crop production and quality.
Related collections
Most cited references160
- Record: found
- Abstract: found
- Article: not found
Efficient In Vivo Genome Editing Using RNA-Guided Nucleases

- Record: found
- Abstract: found
- Article: found
Efficient design and assembly of custom TALEN and other TAL effector-based constructs for DNA targeting
- Record: found
- Abstract: found
- Article: not found