- Record: found
- Abstract: found
- Article: found
Unexpected Hepatotoxicity of Rifampin and Saquinavir/Ritonavir in Healthy Male Volunteers
Read this article at
Abstract
Objectives
Rifampin is a potent inducer of the cytochrome P450 3A4 isoenzyme (CYP3A4) that metabolizes most protease inhibitor (PI) antiretrovirals. This study was designed to evaluate the steady-state pharmacokinetics and tolerability of the coadministration of the PIs saquinavir and ritonavir (a CYP3A4 inhibitor used as a pharmacoenhancer of other PIs) and rifampin when coadministered in healthy HIV-negative volunteers.
Methods
In an open-label, randomized, one sequence, two-period crossover study involving 28 healthy HIV-negative volunteers, arm 1 was randomized to receive saquinavir/ritonavir 1000/100 mg twice daily while arm 2 received rifampin 600 mg once daily for 14 days. Both arms were then to receive concomitant saquinavir/ritonavir and rifampin for 2 additional weeks. Vital signs, electrocardiography, laboratory analyses, and blood levels of total saquinavir, ritonavir, rifampin, and desacetyl-rifampin, the primary metabolite of rifampin, were measured.
Results
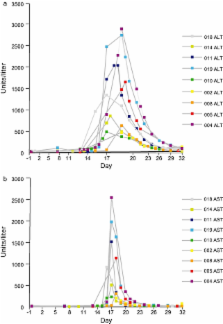
In arm 1, 10/14 (71%) and, in arm 2, 11/14 (79%) participants completed the first study phase; eight participants in arm 1 and nine in arm 2 went on to receive both saquinavir/ritonavir and rifampin. Following substantial elevations (≥ grade 2) in hepatic transaminases in participants receiving the coadministered agents, the study was discontinued prematurely. Two participants in arm 1 displayed moderate elevations after five and four doses of rifampin, respectively. In arm 2, all participants experienced severe elevations within 4 days of initiating saquinavir/ritonavir. Clinical symptoms (e.g., nausea, vomiting, abdominal pain, and headache) were more common and severe in arm 2. Clinical symptoms abated and transaminases normalized following drug discontinuation. Limited pharmacokinetic data suggest a possible relationship between transaminase elevation and elevated rifampin and desacetyl-rifampin concentrations.
Related collections
Most cited references34
- Record: found
- Abstract: found
- Article: not found
Randomised trial of isoniazid versus rifampicin and pyrazinamide for prevention of tuberculosis in HIV-1 infection.
- Record: found
- Abstract: found
- Article: not found
A comprehensive hepatic safety analysis of nevirapine in different populations of HIV infected patients.
- Record: found
- Abstract: found
- Article: not found