- Record: found
- Abstract: found
- Article: found
Disappearance of Seasonal Respiratory Viruses in Children Under Two Years Old During COVID-19 Pandemic: A Monocentric Retrospective Study in Milan, Italy
Read this article at
Abstract
Background: The containment measures adopted during COVID-19 pandemic have influenced the epidemiology of other respiratory viruses.
Aim: We analyzed the modification of the incidence and etiology of lower respiratory tract infections (LRTIs) in young children during COVID-19 pandemic.
Methods: Case series of all children under 2 years old hospitalized at a tertiary care Hospital in the Center of Milan, Italy diagnosed with LRTIs in three consecutive winter seasons (from the 1st of November to the last day of February in 2018/2019, 2019/2020 and 2020/2021). We compared the number of hospitalizations and viral detections in the 2020/2021 with the average of 2018/2019 and 2019/2020 (pre-COVID-19) using the Poisson distribution.
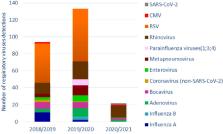
Results: we enrolled 178 patients (66 from 2018/2019, 96 from 2019/2020, 16 from 2020/2021) 94 males (53%) and 84 females (47%), with a median (IQR) age of 5 (2–13) months. The number of hospitalizations during the 2020/2021 season was 80% lower than the average of the pre-COVID-19 seasons (16 vs. 81, p< 0.001). Overall, 171 (96%) patient's nasopharyngeal aspirate (NPA) detected at least one virus (110, 64%, single-detection, 61, 36%, co-detections). In 2020/2021 we observed the disappearance of Respiratory Syncytial virus (0 vs. 54, p < 0.001), Influenza virus (0 vs. 6.5, p = 0.002), Metapneumovirus (0 vs. 8, p < 0.001), Parainfluenza viruses (0 vs. 3.5, p = 0.03) and a significant reduction of Adenovirus (2 vs. 7, p = 0.03), Bocavirus (2 vs. 7.5, p = 0.02) and Enterovirus (1 vs. 5, p = 0.04). No significant difference was found for Rhinoviruses (14 cases vs. 17, p = 0.2), other Coronaviruses (0 vs. 2, p = 0.1), and Cytomegalovirus (1 vs. 1, p = 0.7).
Conclusions: We observed a striking reduction in hospitalizations due to LRTIs and a modification of the etiology, with enveloped viruses mainly affected.
Related collections
Most cited references36
- Record: found
- Abstract: found
- Article: not found
Safety and Efficacy of the BNT162b2 mRNA Covid-19 Vaccine
- Record: found
- Abstract: found
- Article: found
Physical distancing, face masks, and eye protection to prevent person-to-person transmission of SARS-CoV-2 and COVID-19: a systematic review and meta-analysis
- Record: found
- Abstract: found
- Article: not found