- Record: found
- Abstract: found
- Article: found
Th1-Th17 Cells Mediate Protective Adaptive Immunity against Staphylococcus aureus and Candida albicans Infection in Mice
Read this article at
Abstract
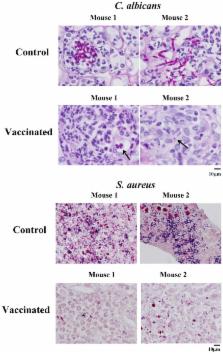
We sought to define protective mechanisms of immunity to Staphylococcus aureus and Candida albicans bloodstream infections in mice immunized with the recombinant N-terminus of Als3p (rAls3p-N) vaccine plus aluminum hydroxide (Al(OH 3) adjuvant, or adjuvant controls. Deficiency of IFN-γ but not IL-17A enhanced susceptibility of control mice to both infections. However, vaccine-induced protective immunity against both infections required CD4+ T-cell-derived IFN-γ and IL-17A, and functional phagocytic effectors. Vaccination primed Th1, Th17, and Th1/17 lymphocytes, which produced pro-inflammatory cytokines that enhanced phagocytic killing of both organisms. Vaccinated, infected mice had increased IFN-γ, IL-17, and KC, increased neutrophil influx, and decreased organism burden in tissues. In summary, rAls3p-N vaccination induced a Th1/Th17 response, resulting in recruitment and activation of phagocytes at sites of infection, and more effective clearance of S. aureus and C. albicans from tissues. Thus, vaccine-mediated adaptive immunity can protect against both infections by targeting microbes for destruction by innate effectors.
Author Summary
The bacterium Staphylococcus aureus and the fungus Candida are the second and third leading cause of bloodstream infections in hospitalized patients. A vaccine to prevent such infections would be of enormous public health benefit. The leading hypothesis to explain why vaccines have not been successfully developed against these infections is that the microbes causing the infections are highly complex, and use multiple weapons (so-called “virulence factors”) to cause disease in humans. Therefore, a vaccine targeting either infection would have to neutralize many of these virulence factors at the same time. We have been developing a vaccine that simultaneously targets both types of infections. Our vaccine is based on a single virulence factor used by Candida, which has a similar shape to virulence factors used by S. aureus. In the current study, we report that our vaccine induces specialized cells in the immune system to more effectively call in reinforcements to kill the organisms. These data demonstrate that vaccines against both organisms can be developed even if they do not work by neutralizing multiple virulence factors, and therefore open the door to a far wider array of vaccine types against both infections.
Related collections
Most cited references54
- Record: found
- Abstract: found
- Article: not found
IL-23 and IL-17 in the establishment of protective pulmonary CD4+ T cell responses after vaccination and during Mycobacterium tuberculosis challenge.
- Record: found
- Abstract: found
- Article: not found
Requirement of Interleukin 17 Receptor Signaling for Lung Cxc Chemokine and Granulocyte Colony-Stimulating Factor Expression, Neutrophil Recruitment, and Host Defense
- Record: found
- Abstract: found
- Article: found