- Record: found
- Abstract: found
- Article: found
Purpurin Suppresses Candida albicans Biofilm Formation and Hyphal Development
Read this article at
Abstract
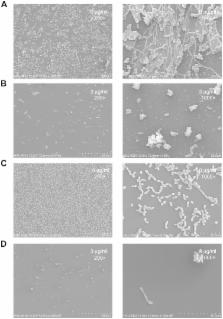
A striking and clinically relevant virulence trait of the human fungal pathogen Candida albicans is its ability to grow and switch reversibly among different morphological forms. Inhibition of yeast-to-hypha transition in C. albicans represents a new paradigm for antifungal intervention. We have previously demonstrated the novel antifungal activity of purpurin against Candida fungi. In this study, we extended our investigation by examining the in vitro effect of purpurin on C. albicans morphogenesis and biofilms. The susceptibility of C. albicans biofilms to purpurin was examined quantitatively by 2,3-bis(2-methoxy-4-nitro-5-sulfo-phenyl)-2H-tetrazolium-5-carboxanilide reduction assay. Hyphal formation and biofilm ultrastructure were examined qualitatively by scanning electron microscopy (SEM). Quantitative reverse transcription-PCR (qRT-PCR) was used to evaluate the expression of hypha-specific genes and hyphal regulator in purpurin-treated fungal cells. The results showed that, at sub-lethal concentration (3 µg/ml), purpurin blocked the yeast-to-hypha transition under hypha-inducing conditions. Purpurin also inhibited C. albicans biofilm formation and reduced the metabolic activity of mature biofilms in a concentration-dependent manner. SEM images showed that purpurin-treated C. albicans biofilms were scanty and exclusively consisted of aggregates of blastospores. qRT-PCR analyses indicated that purpurin downregulated the expression of hypha-specific genes ( ALS3, ECE1, HWP1, HYR1) and the hyphal regulator RAS1. The data strongly suggested that purpurin suppressed C. albicans morphogenesis and caused distorted biofilm formation. By virtue of the ability to block these two virulence traits in C. albicans, purpurin may represent a potential candidate that deserves further investigations in the development of antifungal strategies against this notorious human fungal pathogen in vivo.
Related collections
Most cited references35
- Record: found
- Abstract: found
- Article: not found
Nonfilamentous C. albicans mutants are avirulent.
- Record: found
- Abstract: found
- Article: not found
Medicinal plants and antimicrobial activity.
- Record: found
- Abstract: found
- Article: not found