- Record: found
- Abstract: found
- Article: found
Development and evaluation of latex agglutination test coating with recombinant antigen, LipL32 for serodiagnosis of human leptospirosis
Read this article at
Abstract
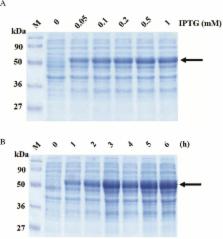
Leptospirosis is a widespread zoonotic disease caused by Leptospira interrogans. Symptoms of disease range from mild symptoms to serious complications including, jaundice, pulmonary hemorrhage, renal and hepatic failure, which may prove fatal. Clinical presentations of this disease are similar with other febrile illness. Therefore, rapid and appropriated laboratory diagnostic tests are needed to aid clinical case identification. As these reasons, objective of this study is to develop and evaluate a simple latex agglutination test coating with recombinant leptospiral antigens, LipL32 for serodiagnosis of human leptospirosis. Firstly, lipl32 gene was amplified from genomic DNA of Leptospira interogans serovar Pyrogenes. Then PCR product of lipl32 gene was ligated with pGEX-2T plasmid, generating pGRK32 recombinant plasmid. Recombinant GST-LipL32 protein was overexpressed and subsequently purified by using Glutathione-Agarose Resin. Recombinant GST-Lipl32 protein was coated on latex beads for development latex agglutination test (LAT). The relative sensitivity, specificity and accuracy of the developed LAT were compared with indirect immunofluorescences assay (IFA) for detection of anti-leptospiral antibodies in 30 human leptospirosis samples, 30 healthy blood donor samples, 10 dengue fever positive samples, 10 scrub typhus positive samples, and 10 melioidosis samples. Results showed that the developed LAT showed sensitivity, specificity and accuracy: 66.66%, 86.66%, and 80.00%, respectively, comparing with IFA method. Moreover, Kappa analysis showed agreement rate of the two methods were 0.421. It concluded that our developed gave compatible result with IFA. Additionally, Our LAT are simple, rapid and suitable for detection in the field. However, for better sensitivity, diagnostic specificity, positive predictive value, negative predictive value, accuracy and Cohen’s kappa comparison should be done in larger amounts of sera samples.