- Record: found
- Abstract: found
- Article: found
Can Polarity-Inverted Surfactants Self-Assemble in Nonpolar Solvents?

Read this article at
Abstract
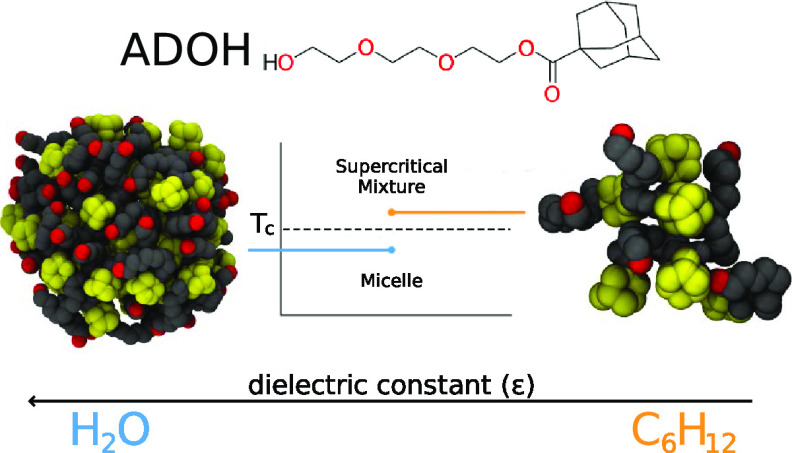
We investigate the self-assembly process of a surfactant with inverted polarity in water and cyclohexane using both all-atom and coarse-grained hybrid particle-field molecular dynamics simulations. Unlike conventional surfactants, the molecule under study, proposed in a recent experiment, is formed by a rigid and compact hydrophobic adamantane moiety, and a long and floppy triethylene glycol tail. In water, we report the formation of stable inverted micelles with the adamantane heads grouping together into a hydrophobic core and the tails forming hydrogen bonds with water. By contrast, microsecond simulations do not provide evidence of stable micelle formation in cyclohexane. Validating the computational results by comparison with experimental diffusion constant and small-angle X-ray scattering intensity, we show that at laboratory thermodynamic conditions the mixture resides in the supercritical region of the phase diagram, where aggregated and free surfactant states coexist in solution. Our simulations also provide indications as to how to escape this region to produce thermodynamically stable micellar aggregates.
Related collections
Most cited references44
- Record: found
- Abstract: not found
- Article: not found
GROMACS: High performance molecular simulations through multi-level parallelism from laptops to supercomputers
- Record: found
- Abstract: not found
- Article: not found