- Record: found
- Abstract: found
- Article: found
Clinical evaluation of efficacy and safety of cyclosporine (Imusporin) in renal transplant patients with stable graft function maintained on neoral or bioral
Read this article at
Abstract
Objective:
Previous pharmacokinetic studies have demonstrated bioequivalence of Imusporin (microemulsion preparation of cyclosporine, Cipla) to the innovator product Neoral (Novartis, Switzerland). This study was done to evaluate the clinical efficacy and safety of Imusporin in patients who have already undergone renal transplant and have stable graft function maintained on cyclosporine preparation other than Imusporin.
Materials and Methods:
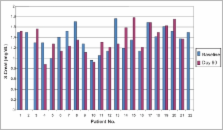
Twenty-two renal allograft recipients (mean age of 31.77 years, range 18-53 years), with stable graft function, previously on Neoral or Bioral were switched over to Imusporin after recording their relevant baseline clinical and biochemical parameters. These were repeated on 1, 4, 7, 15, 30 and 90 days after the start of therapy. Change in dosage required to maintain C2 levels at each visit were analyzed by paired sample t-test. Safety of the drug was assessed by the type and severity of adverse events developed during the therapy. Cost analysis was done assuming an average maintenance immunosuppression dose of 150 mg/day of cyclosporine.
Results:
Twenty-one patients completed the study. One patient was lost to follow-up. Mean C2 value before switchover was 894 ± 208 ng/ml, which was not significantly different from the mean values of C2 after switchover therapy ( P>0.30). Change in dosage required to maintain C2 levels was not significantly different from the baseline dose of 2.34 mg/ kg body weight ( P>0.1). No patient developed graft rejection after switchover therapy at a median follow-up of 16 months (14-18 months). Mean baseline SCr was similar to SCr at day 90 (1.38 vs. 1.37 mg/dl, P=0.930). No severe adverse events were reported. Mild side-effects included headache (4), somnolence (2), dry mouth (5) and generalized fatigue (6). Use of Imusporin (Cipla, India) results in an annual savings of Rs. 19892 over Neoral (Novartis, Switzerland) and Rs. 2263 over Bioral (Panacea Biotech, India).
Related collections
Most cited references8
- Record: found
- Abstract: found
- Article: not found
Long-term efficacy and safety of cyclosporine in renal-transplant recipients.
- Record: found
- Abstract: found
- Article: not found
A pharmacokinetic and clinical review of the potential clinical impact of using different formulations of cyclosporin A. Berlin, Germany, November 19, 2001.
- Record: found
- Abstract: found
- Article: not found