- Record: found
- Abstract: found
- Article: found
18F-Labeled, PSMA-Targeted Radiotracers: Leveraging the Advantages of Radiofluorination for Prostate Cancer Molecular Imaging
Read this article at
Abstract
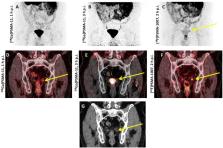
Prostate-specific membrane antigen (PSMA)-targeted PET imaging for prostate cancer with 68Ga-labeled compounds has rapidly become adopted as part of routine clinical care in many parts of the world. However, recent years have witnessed the start of a shift from 68Ga- to 18F-labeled PSMA-targeted compounds. The latter imaging agents have several key advantages, which may lay the groundwork for an even more widespread adoption into the clinic. First, facilitated delivery from distant suppliers expands the availability of PET radiopharmaceuticals in smaller hospitals operating a PET center but lacking the patient volume to justify an onsite 68Ge/ 68Ga generator. Thus, such an approach meets the increasing demand for PSMA-targeted PET imaging in areas with lower population density and may even lead to cost-savings compared to in-house production. Moreover, 18F-labeled radiotracers have a higher positron yield and lower positron energy, which in turn decreases image noise, improves contrast resolution, and maximizes the likelihood of detecting subtle lesions. In addition, the longer half-life of 110 min allows for improved delayed imaging protocols and flexibility in study design, which may further increase diagnostic accuracy. Moreover, such compounds can be distributed to sites which are not allowed to produce radiotracers on-site due to regulatory issues or to centers without access to a cyclotron. In light of these advantageous characteristics, 18F-labeled PSMA-targeted PET radiotracers may play an important role in both optimizing this transformative imaging modality and making it widely available. We have aimed to provide a concise overview of emerging 18F-labeled PSMA-targeted radiotracers undergoing active clinical development. Given the wide array of available radiotracers, comparative studies are needed to firmly establish the role of the available 18F-labeled compounds in the field of molecular PCa imaging, preferably in different clinical scenarios.
Related collections
Most cited references76

- Record: found
- Abstract: found
- Article: not found
[ 177 Lu]-PSMA-617 radionuclide treatment in patients with metastatic castration-resistant prostate cancer (LuPSMA trial): a single-centre, single-arm, phase 2 study
- Record: found
- Abstract: found
- Article: not found