- Record: found
- Abstract: found
- Article: found
SERS monitoring of photoinduced-enhanced oxidative stress amplifier on Au@carbon dots for tumor catalytic therapy
Read this article at
Abstract
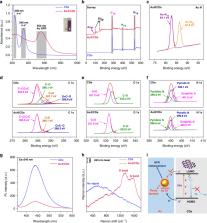
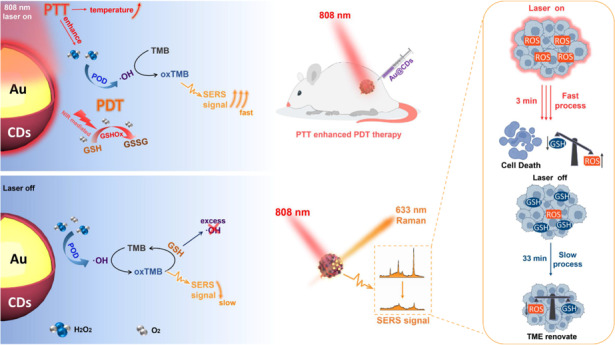
Currently, artificial enzymes-based photodynamic therapy (PDT) is attractive due to its efficient capacity to change the immunosuppressive tumor microenvironment (TME). It is of great significance to study the therapeutic mechanism of novel artificial enzymes in TME through a monitoring strategy and improve the therapeutic effect. In this study, Au@carbon dots (Au@CDs) nanohybrids with a core-shell structure are synthesized, which not only exhibit tunable enzyme-mimicking activity under near-infrared (NIR) light, but also excellent surface-enhanced Raman scattering (SERS) properties. Therefore, Au@CDs show a good capability for monitoring NIR-photoinduced peroxidase-like catalytic processes via a SERS strategy in tumor. Moreover, the Au@CDs deplete glutathione with the cascade catalyzed reactions, thus elevating intratumor oxidative stress amplifying the reactive oxygen species damage based on the NIR-photoinduced enhanced peroxidase and glutathione oxidase-like activities, showing excellent and fast PDT therapeutic effect promoted by photothermal property in 3 min, finally leading to apoptosis in cancer cells. Through SERS monitoring, it is further found that after removing the NIR light source for 33 min, the reactive oxygen species (ROS) activity of the TME is counteracted and eliminated due to the presence of glutathione. This work presents a guidance to rationally design of artificial enzyme for ROS-involved therapeutic strategies and a new spectroscopic tool to evaluate the tumor catalytic therapy.
Abstract
Core-shell structured Au@carbon dots are fabricated to present a photoinduced-enhanced oxidative stress amplification, which can be efficiently used for tumor catalytic therapy by surface-enhanced Raman scattering technique.
Related collections
Most cited references49
- Record: found
- Abstract: found
- Article: not found
Free radicals and antioxidants in normal physiological functions and human disease.
- Record: found
- Abstract: found
- Article: not found
Targeting cancer cells by ROS-mediated mechanisms: a radical therapeutic approach?
- Record: found
- Abstract: not found
- Article: not found