- Record: found
- Abstract: found
- Article: found
Molecular dissection of the male germ cell lineage identifies putative spermatogonial stem cells in rhesus macaques
Read this article at
Abstract
BACKGROUND
The spermatogonial stem cell (SSC) pool in the testes of non-human primates is poorly defined.
METHODS
To begin characterizing SSCs in rhesus macaque testes, we employed fluorescence-activated cell sorting (FACS), a xenotransplant bioassay and immunohistochemical methods and correlated our findings with classical descriptions of germ cell nuclear morphology (i.e. A dark and A pale spermatogonia).
RESULTS
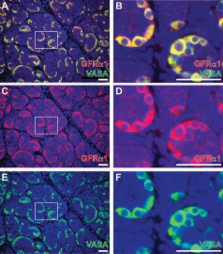
FACS analysis identified a THY-1 + fraction of rhesus testis cells that was enriched for consensus SSC markers (i.e. PLZF, GFRα1) and exhibited enhanced colonizing activity upon transplantation to nude mouse testes. We observed a substantial conservation of spermatogonial markers from mice to monkeys [PLZF, GFRα1, Neurogenin 3 (NGN3), cKIT]. Assuming that molecular characteristics correlate with function, the pool of putative SSCs (THY-1 +, PLZF +, GFRα1 +, NGN3 +/−, cKIT −) comprises most A dark and A pale and is considerably larger in primates than in rodents. It is noteworthy that the majority of A dark and A pale share a common molecular phenotype, considering their distinct functional classifications as reserve and renewing stem cells, respectively. NGN3 is absent from A dark, but is expressed by some A pale and may mark the transition from undifferentiated (cKIT −) to differentiating (cKIT +) spermatogonia. Finally, the pool of transit-amplifying progenitor spermatogonia (PLZF +, GFRα1 +, NGN3 +, cKIT +/−) is smaller in primates than in rodents.
Related collections
Most cited references66
- Record: found
- Abstract: found
- Article: not found
Essential role of Plzf in maintenance of spermatogonial stem cells.
- Record: found
- Abstract: found
- Article: not found
Plzf is required in adult male germ cells for stem cell self-renewal.
- Record: found
- Abstract: not found
- Article: not found