- Record: found
- Abstract: found
- Article: found
Photodegradation Study of Sertindole by UHPLC-ESI-Q-TOF and Influence of Some Metal Oxide Excipients on the Degradation Process

Read this article at
Abstract
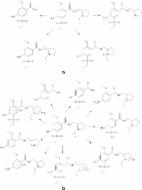
The evaluation of the influence of the excipients present in the pharmaceutical formulations on the drug stability is an important part of quality control of medicines. One of the most commonly applied group of excipients are pigments, such as titanium dioxide or various forms of iron oxides, which are well-known photocatalytic agents. Therefore, the photostability of an atypical antipsychotic drug sertindole and the influence of pigments commonly used in the pharmaceutical formulations (FeOOH, Fe 2O 3, and TiO 2) on this process were studied. The quantitative and qualitative analysis of the process was performed with the use of ultra high pressure liquid chromatography with diode array detection (UHPLC-DAD) system coupled with a high resolution hybrid electrospray ionization quadrupole time-of-flight (ESI-Q-TOF) mass spectrometer. Sertindole turned out to be a highly photolabile molecule. Overall 18 transformation products were found, mainly formed as a consequence of dechlorination, hydroxylation, and dehydrogenation. In all the experiments, except the TiO 2-mediated photocatalysis, the product of chlorine substitution with a hydroxyl group was the major product. The presence of Fe 2O 3 and TiO 2 accelerated the degradation process, while FeOOH served as its inhibitor. The experiments conducted with the use of the pharmaceutical formulations confirmed the catalytic activity of the used excipients. The exploration of the obtained phototransformation profiles with the use of principal component analysis (PCA) revealed that the presence of both iron oxides could influence the qualitative and quantitative aspect of the studied processes. In silico assessment of the properties showed that the transformation products are generally less toxic to rodents, possess lower hERG blocking potential, but could be more mutagenic than the parent molecule.
Related collections
Most cited references14
- Record: found
- Abstract: found
- Article: found
Studies on photodegradation process of psychotropic drugs: a review
- Record: found
- Abstract: found
- Article: not found
A randomized, controlled, dose-ranging trial of sertindole in patients with schizophrenia.
- Record: found
- Abstract: not found
- Article: not found