- Record: found
- Abstract: found
- Article: found
The feasibility of a randomized controlled trial of esophagectomy for esophageal cancer - the ROMIO (Randomized Oesophagectomy: Minimally Invasive or Open) study: protocol for a randomized controlled trial
Read this article at
Abstract
Background
There is a need for evidence of the clinical effectiveness of minimally invasive surgery for the treatment of esophageal cancer, but randomized controlled trials in surgery are often difficult to conduct. The ROMIO (Randomized Open or Minimally Invasive Oesophagectomy) study will establish the feasibility of a main trial which will examine the clinical and cost-effectiveness of minimally invasive and open surgical procedures for the treatment of esophageal cancer.
Methods/Design
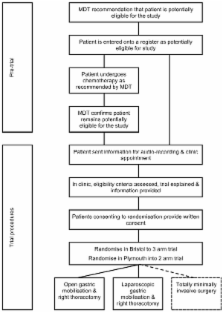
A pilot randomized controlled trial (RCT), in two centers (University Hospitals Bristol NHS Foundation Trust and Plymouth Hospitals NHS Trust) will examine numbers of incident and eligible patients who consent to participate in the ROMIO study. Interventions will include esophagectomy by: (1) open gastric mobilization and right thoracotomy, (2) laparoscopic gastric mobilization and right thoracotomy, and (3) totally minimally invasive surgery (in the Bristol center only). The primary outcomes of the feasibility study will be measures of recruitment, successful development of methods to monitor quality of surgery and fidelity to a surgical protocol, and development of a core outcome set to evaluate esophageal cancer surgery. The study will test patient-reported outcomes measures to assess recovery, methods to blind participants, assessments of surgical morbidity, and methods to capture cost and resource use. ROMIO will integrate methods to monitor and improve recruitment using audio recordings of consultations between recruiting surgeons, nurses, and patients to provide feedback for recruiting staff.
Discussion
The ROMIO study aims to establish efficient methods to undertake a main trial of minimally invasive surgery versus open surgery for esophageal cancer.
Trial registration
The pilot trial has Current Controlled Trials registration number ISRCTN59036820(25/02/2013) at http://www.controlled-trials.com; the ROMIO trial record at that site gives a link to the original version of the study protocol.
Related collections
Most cited references32
- Record: found
- Abstract: found
- Article: not found
Minimally invasive versus open oesophagectomy for patients with oesophageal cancer: a multicentre, open-label, randomised controlled trial.
- Record: found
- Abstract: found
- Article: not found
Extended transthoracic resection compared with limited transhiatal resection for adenocarcinoma of the esophagus.
- Record: found
- Abstract: found
- Article: not found