- Record: found
- Abstract: found
- Article: found
High-Resolution Free-Breathing Quantitative First-Pass Perfusion Cardiac MR Using Dual-Echo Dixon With Spatio-Temporal Acceleration
Read this article at
Abstract
Introduction
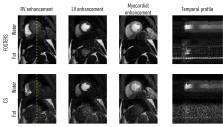
To develop and test the feasibility of free-breathing (FB), high-resolution quantitative first-pass perfusion cardiac MR (FPP-CMR) using dual-echo Dixon (FOSTERS; Fat-water separation for mOtion-corrected Spatio-TEmporally accelerated myocardial peRfuSion).
Materials and Methods
FOSTERS was performed in FB using a dual-saturation single-bolus acquisition with dual-echo Dixon and a dynamically variable Cartesian k-t undersampling (8-fold) approach, with low-rank and sparsity constrained reconstruction, to achieve high-resolution FPP-CMR images. FOSTERS also included automatic in-plane motion estimation and T correction to obtain quantitative myocardial blood flow (MBF) maps. High-resolution (1.6 x 1.6 mm 2) FB FOSTERS was evaluated in eleven patients, during rest, against standard-resolution (2.6 x 2.6 mm 2) 2-fold SENSE-accelerated breath-hold (BH) FPP-CMR. In addition, MBF was computed for FOSTERS and spatial wavelet-based compressed sensing (CS) reconstruction. Two cardiologists scored the image quality (IQ) of FOSTERS, CS, and standard BH FPP-CMR images using a 4-point scale (1–4, non-diagnostic – fully diagnostic).
Results
FOSTERS produced high-quality images without dark-rim and with reduced motion-related artifacts, using an 8x accelerated FB acquisition. FOSTERS and standard BH FPP-CMR exhibited excellent IQ with an average score of 3.5 ± 0.6 and 3.4 ± 0.6 (no statistical difference, p > 0.05), respectively. CS images exhibited severe artifacts and high levels of noise, resulting in an average IQ score of 2.9 ± 0.5. MBF values obtained with FOSTERS presented a lower variance than those obtained with CS.
Related collections
Most cited references55
- Record: found
- Abstract: found
- Article: found
Magnetic Resonance Perfusion or Fractional Flow Reserve in Coronary Disease
- Record: found
- Abstract: found
- Article: not found
Accelerated dynamic MRI exploiting sparsity and low-rank structure: k-t SLR.

- Record: found
- Abstract: found
- Article: found