- Record: found
- Abstract: found
- Article: found
Prevalence and Nature of Medication Errors and Medication-Related Harm Following Discharge from Hospital to Community Settings: A Systematic Review
Read this article at
Abstract
Background
Little is known about the epidemiology of medication errors and medication-related harm following transition from secondary to primary care. This systematic review aims to identify and critically evaluate the available evidence on the prevalence and nature of medication errors and medication-related harm following hospital discharge.
Methods
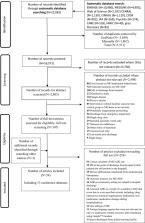
Studies published between January 1990 and March 2019 were searched across ten electronic databases and the grey literature. No restrictions were applied with publication language or patient population studied. Studies were included if they contained data concerning the rate of medication errors, unintentional medication discrepancies, or adverse drug events. Two authors independently extracted study data.
Results
Fifty-four studies were included, most of which were rated as moderate (39/54) or high (7/54) quality. For adult patients, the median rate of medication errors and unintentional medication discrepancies following discharge was 53% [interquartile range 33–60.5] ( n = 5 studies) and 50% [interquartile range 39–76] ( n = 11), respectively. Five studies reported adverse drug reaction rates with a median of 27% [interquartile range 18–40.5] and seven studies reported adverse drug event rates with a median of 19% [interquartile range 16–24]. For paediatric patients, one study reported a medication error rate of 66.3% and another an adverse drug event rate of 9%. Almost a quarter of studies (13/54, 24%) utilised a follow-up period post-discharge of 1 month (range 2–180 days). Drug classes most commonly implicated with adverse drug events were antibiotics, antidiabetics, analgesics and cardiovascular drugs.
Related collections
Most cited references141

- Record: found
- Abstract: found
- Article: found
Unwanted incidents during transition of geriatric patients from hospital to home: a prospective observational study
- Record: found
- Abstract: not found
- Article: not found
Incidence of Adverse Drug Events and Potential Adverse Drug Events
- Record: found
- Abstract: found
- Article: not found